-
Position Function Location Job description
-
Elpiscience Presents Phase I Data for First-in-Class ES014 (CD39/TGF-βBispecific) in Oral Session at ESMO AsiaJob descriptionJob description
On December 5, 2025, Elpiscience, a clinical-stage biopharmaceutical company dedicated to developing innovative immunotherapies, presented phase I clinical trial results for its internally developed bispecific antibody, ES014, in an oral presentation at the ESMO Asia Congress.
ES014 is a first-in-class CD39/TGF-βbispecific antibody developed by Elpiscience, and the first molecule globally to enter the clinical stage that simultaneously targets the CD39-adenosine and TGF-βpathways—two critical immunosuppressive mechanisms in the tumor microenvironment. ES014 holds the potential for treating a variety of solid tumors. Preclinical studies have demonstrated a favorable safety profile and antitumor activity, indicating its potential both as a monotherapy and in combination with other agents such as chemotherapy and PD-1 checkpoint inhibitors.
Elpiscience announced that the first-in-human Phase I clinical results of ES014, the world’s first-in-class CD39/TGF-βbispecific antibody developed in-house, will be presented as an oral presentation at the Proffered Paper Session during ESMO Asia 2025, scheduled to take place on December 5, 2025, in Singapore.
The presented data are based on an ongoing open-label, multi-center phase I clinical trial of ES014 in China (comprising the dose escalation part and the cohort expansion part), designed to evaluate the safety and efficacy of ES014 monotherapy in patients with advanced solid tumors. Patient enrollment was completed in June 2025. The dose escalation part included five dose levels (20 mg to 1400 mg, administered every two weeks). The cohort expansion part enrolled patients with various tumor types, including non-small cell lung cancer (NSCLC), gastrointestinal stromal tumors (GIST), and desmoid tumors (DT). The primary endpoints were safety and tolerability, and the secondary endpoints included pharmacokinetics, immunogenicity, and preliminary anti-tumor activity.
The study enrolled a total of 75 patients, including 43 patients with NSCLC (57.3%), 10 patients with GIST (13.3%), 5 patients with DT (6.7%), and 17 patients with other solid tumors. 45% of patients had received three or more prior lines of therapy. The median age was 64 years. 54 patients were male (72%) and 21 were female (28%).
ES014 demonstrated a favorable safety profile with no observed DLTs and most adverse events were mild.
Results showed that ES014 has a very favorable safety profile, with no dose-limiting toxicities (DLTs) observed. Most adverse events were mild (Grade 1 or 2). The most common treatment-related adverse events (TRAEs) included anaemia, rash, and pruritus, all of which are known to be associated with TGF-β inhibition. Grade 3 or higher TRAEs occurred in 16% of patients, and there were no treatment-emergent adverse events (TEAEs) leading to death.
ES014 demonstrated monotherapy efficacy across multiple solid tumors, achieving 40% ORR in Desmoid Tumors (DT).
ES014 exhibited significant monotherapy activity in patients with DT. Among the 5 DT patients treated with ES014, 2 achieved partial response (PR) and 3 achieved stable disease (SD), resulting in an objective response rate (ORR) of 40% and a disease control rate (DCR) of 100%. Both patients who achieved PR remain on treatment. As a rare tumor characterized by high local aggressiveness and recurrence rates, DT currently lacks effective and safe treatment options, representing a significant unmet clinical need. With its superior efficacy and safety profile, ES014 holds promise to bring new hope to DT patients.
In the NSCLC cohort, the study found a significant correlation between baseline CD39 combined positive score (CPS) and clinical benefit. Among the 21 response-evaluable NSCLC patients treated at the recommended doses who had high baseline CD39 expression baseline CD39 CPS ≥ 5), 1 achieved PR and 10 achieved SD, resulting in a DCR of 52.4%. By contrast, among the 8 response-evaluable NSCLC patients treated at the recommended doses who had low baseline CD39 expression (CD39 CPS < 5), only 1 achieved SD, resulting in a DCR of 12.5%. These results demonstrate a significant correlation between baseline CD39 expression levels and treatment response in the NSCLC cohort, supporting CD39 as a potential biomarker for selecting NSCLC patients for ES014 treatment.
ES014 also showed positive signals of clinical benefit in the gastrointestinal stromal tumor (GIST) patient population. Among 10 GIST patients treated with ES014, 1 achieved PR and 6 achieved SD, resulting in a DCR of 70%. Notably, in the subgroup of 4 wild-type GIST patients, tumor shrinkage was observed in 2 patients, with 1 achieving PR. These results suggest that ES014 has the potential to provide a new treatment option for GIST, particularly for patients with wild-type GIST.
Professor Shun Lu, Academic Leader of the Department of Oncology at Shanghai Chest Hospital and Director of the Shanghai Lung Tumor Clinical Medical Center noted,“ES014 innovatively blocks two key immunosuppressive pathways, CD39 and TGF-β, providing a novel strategy for clinical oncology treatment. The results of this phase I study show that ES014 has a favorable safety profile and clear pharmacodynamic characteristics. Preliminary efficacy signals were observed in multiple tumor types, including non-small cell lung cancer, gastrointestinal stromal tumors, and desmoid tumors, which is very encouraging. This study fully demonstrates ES014's strong potential for treating various solid tumors and is expected to bring a novel and effective treatment option to these patients."
Dr. Xiaohui Ji, Co-founder and CEO of Elpiscience noted,“ES014 is a significant innovation resulting from Elpiscience’s deep commitment to immunology. The clinical progress announced at ESMO Asia further validates the innovation of ES014 as a first-in-class asset and its clinical application potential in the field of solid tumors. We are delighted to see ES014 safely and effectively addressing unmet clinical needs for cancer patients, especially for diseases like desmoid tumors that lack standard treatments. In the future, Elpiscience will accelerate the subsequent development of ES014 to bring breakthrough treatment solutions to more cancer patients.”
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Preview | Oral Presentation at ESMO Asia 2025: First-in-Human Results of ES014, the World’s First CD39/TGF-β Bispecific Antibody, as Monotherapy in Advanced Solid Tumors to Be UnveiledJob descriptionJob description
Elpiscience announced that the first-in-human Phase I clinical results of ES014, the world’s first-in-class CD39/TGF-β bispecific antibody developed in-house, will be presented as an oral presentation at the Proffered Paper Session during ESMO Asia 2025, scheduled to take place on December 5, 2025, in Singapore.
ES014 is the first-in-class and first clinical-stage CD39/TGFβ bsAb worldwide independently developed by Elpiscience, which targets two different immunosuppressive pathways in the tumor microenvironment (TME) : the CD39–adenosine pathway and the TGF-β pathway. ES014 has demonstrated favorable safety and antitumor activity in preclinical studies, supporting its potential to treat a wide range of solid tumors. In December 2022, the company received IND approval from China’s National Medical Products Administration (NMPA) to initiate the Phase I clinical trial. The ongoing study evaluates ES014 as monotherapy in patients with advanced solid tumors and has completed patient enrollment, including dose escalation and expansion cohorts.
Data from the Phase I study, to be presented in the oral presentation at ESMO Asia 2025, shows that ES014 has exhibited a favorable safety profile and encouraging antitumor activity across multiple tumor types, including non-small cell lung cancer (NSCLC), Desmoid Tumors(DT), and gastrointestinal stromal tumors (GIST).
The selection of ES014 for an oral presentation in the Proffered Paper Session highlights its strong potential in addressing CD39-high solid tumors and provides important clinical evidence to support further development of ES014.
Presentation Details:
- Presentation Title: First-in-human study of ES014, a bispecific antibody targeting CD39 and TGF-β as monotherapy in patients with advanced solid tumors
- Presentation Number: 159O
- Speaker: Zhen Zhou, M.D., Shanghai Lung Cancer Center, Shanghai Chest Hospital, Shanghai Jiaotong University, School of Medicine
- Session: Proffered Paper Session: Developmental therapeutics and precision medicine
- Date: December 5, 2025, 11:25–11:35
- Location: Hall 401, Suntec Singapore, Singapore
About ESMO Asia
The ESMO Asia Congress, organized by the European Society for Medical Oncology (ESMO), is one of the most influential oncology events in the Asia-Pacific region, dedicated to advancing cancer research and clinical practice across Asia.
The ESMO Asia Congress 2025 will take place from December 5 to 7 in Singapore. The meeting will bring together leading experts to discuss cutting-edge topics including precision medicine, biomarkers, innovative cancer therapies, and cancers with high prevalence in the Asia-Pacific region, with in-depth presentations of the year’s most significant research breakthroughs.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Highlight Recap | Elpiscience Showcases Three Innovative Research Studies at SITC 2025, Demonstrating Original Innovation on the Global Academic StageJob descriptionJob description
From November 5 to 9, the 40th Annual Meeting of the Society for Immunotherapy of Cancer (SITC) was grandly held at National Harbor, Maryland, USA. As one of the world’s largest and most influential academic congresses in cancer immunotherapy, Elpiscience was selected to present for the fifth consecutive year, showcasing three of its latest research achievements through poster presentations. Key highlights included:
- Bispecific Myeloid Engager (BiME) antibodies that harness the DC–CD8⁺ T cell axis to generate durable antitumor immunity;
- ES009, a differentiated and potentially best-in-class LILRB2 inhibitor, demonstrating favorable safety, tolerability, PK/PD, and preliminary antitumor activity in a first-in-human Phase 1 study;
- ES038, a novel NK cell engager (NKCE) targeting LILRB4 via dual engagement of NKG2A and NKG2C, showing strong therapeutic potential for AML.
The three scientific advances from Elpiscience drew significant attention at the conference, demonstrating strong recognition from the international scientific community and further highlighting the company’s innovative strengths and leadership in immuno-oncology. Moving forward, Elpiscience will continue to focus on first-in-class innovation in tumor immunology, accelerate clinical translation, and bring innovative therapies from China to patients worldwide earlier.
Key Research Highlights Presented at SITC 2025:
- Bispecific Myeloid Engager (BiME) Antibodies Harness the DC–CD8⁺ T Cell Axis to Drive Durable Antitumor Immunity
Abstract No.: 1168
Research Overview:
ES004-B5 is a pan-allelic anti-human SIRPα antibody developed by Elpiscience, which potently blocks the SIRPα-CD47 interaction and enhances macrophage phagocytosis when combined with anti-TAA(tumor-associated antigens) IgG1 therapies. Structural analysis indicates that ES004-B5 can potentially block both trans (CD47-SIRPα) and cis (SIRPα-CD18) inhibitory pathways in macrophages. To further unlock its therapeutic value, the research team generated a series of bispecific myeloid engagers (BiMEs) targeting both SIRPα and TAAs. These molecules significantly enhanced macrophage and dendritic-cell phagocytosis, promoted CD8⁺ T cell activation, and demonstrated potent antitumor efficacy in multiple in vivo models. These BiMEs markedly enhanced macrophage/DC phagocytic activity and promoted CD8⁺ T cell activation, leading to robust anti-tumor effects in multiple murine tumor models. Further research demonstrates that BiMEs primarily act through the dendritic cell (DC)-CD8⁺ T cell axis, resulting in potent and durable anti-tumor immune memory. This study further uncovers the antitumor potential of myeloid cells, providing a new strategy for myeloid-targeted therapy.
Key Findings:
- Elpiscience’s anti-SIRPαand BiME molecules recognize a unique epitope overlapping both CD47- and CD18- binding sites on SIRPα
- BiME activates macrophages and DCs both in vitro and in vivo
- BiME promotes CD8+ T enrichment in TME in vivo
- BiME drives tumor inhibition primarily via the DC-CD8⁺ T axis
- BiME induces robust and durable antitumor immune memory
- A first-in-human study evaluating the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of the LILRB2 inhibitor ES009 in patients with advanced solid tumors
Abstract No.: 596
Research Overview:
ES009 is a differentiated mAb targeting LILRB2 with best-in-class potential. By targeting and binding to LILRB2, ES009 reprograms immunosuppressive M2 macrophages into pro-inflammatory M1 macrophages, alleviates M2-mediated T cell suppression, and reshapes the immunosuppressive tumor microenvironment into one more conducive to anti-tumor immunity.
This report presents the results of a first-in-human Phase I clinical study on ES009 (Clinical Trial Number: NCT06007482). The trial is an open-label, multicenter, phase 1 study conducted in Australia to evaluate the safety, tolerability, PK, PD, and preliminary antitumor activity of ES009 in patients with advanced solid tumors who had received and progressed on or were intolerant to standard therapies. Twelve patients—including colorectal cancer, cholangiocarcinoma, NSCLC, ovarian cancer, and submandibular gland cancer—were enrolled. ES009 was administered intravenously every three weeks at doses ranging from 30 to 1600 mg. The DLT assessment period was 21 days.
Key Findings:
- ES009 demonstrated favorable safety and tolerability in patients with advanced solid tumors, with no DLTs observed and the maximum tolerated dose (MTD) not reached.
-At dose levels ≥ 300 mg, ES009 exhibited desirable pharmacokinetic and pharmacodynamic properties.
- Among 11 evaluable patients, 8 patients achieved stable disease as their best overall response, resulting in a disease control rate (DCR) of 72.7%. The median progression-free survival was 2.8 months
- As a monotherapy, ES009 showed preliminary antitumor activity with good safety and tolerability, supporting further clinical development
- ES038, a novel NKCE Molecule Targeting LILRB4 in AML via Dual Engagement of NKG2A and NKG2C
Abstract No.: 435
Research Overview:
Natural killer cell engagers (NKCEs) offer a versatile platform to enhance NK cell-mediated r cytotoxicity. However, conventional NKCEs often fall short in fully activating NK cell due to inhibitory signals such as NKG2A. In addition, AML, particularly in relapsed or refractory cases, remains a significant clinical challenge. LILRB4 has emerged as one of the highly promising new therapeutic targets.
ES038, a novel NKCE that targets LILRB4 on AML cells while simultaneously blocking inhibitory (NKG2A) and stimulating activating (NKG2C) receptors on NK cells, this dual-targeting strategy enhances NK cell activation and improves their therapeutic potential.
Key Findings:
- ES038 is a NK cell engager targeting LILRB4 via dual engagement of NKG2A and NKG2C
- Dual engagement of NKG2A and 2C exhibit superior activity than NKG2A alone
- Dual engagement of NKG2A and 2C could modulate NKG2A+NKG2C+ cell population with stronger IFNγ% and CD107a% expression
- NK cells from AML patients treated with ES038 can kill LILRB4-expressing cells
- By coordinately modulating NKG2A and NKG2C pathways, ES038 represents a novel dual-targeting immunotherapy to break through the therapeutic bottleneck of AML
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Showcase Three Innovative Research Studies at the SITC 2025 Annual MeetingJob descriptionJob description
Elpiscience Biopharmaceuticals today announced that it will showcase three cutting-edge research studies through poster presentations at the 40th Annual Meeting of the Society for Immunotherapy of Cancer (SITC 2025), taking place November 5–9, 2025, in National Harbor, Maryland, USA. The posters involve the company‘s internally developed Bispecific Macrophage Engager (BiME®), the LILRB2-targeting monoclonal antibody ES009, and the bispecific NK cell engager (NKCE) ES038.
The SITC Annual Meeting is the world’s largest international conference dedicated to cancer immunotherapy. As one of the most influential and professional academic conferences globally, it gathers thousands of industry leaders and representatives from academia, regulatory agencies, and government institutions worldwide each year to exchange cutting-edge results and discuss future directions. The 2024 SITC meeting attracted more than 6,000 participants worldwide.
Elpiscience has been selected to participate in SITC Annual Meeting for five consecutive years, highlighting the company’s independent innovation capabilities and significant progress in tumor immunology research. This year, the BiME® study was recognized among the “Top 150 Abstracts” from thousands of selected global submissions, further validating the international academic recognition of BiME®'s originality and promising potential in solid tumor treatment.
Details of Elpiscience’s posters at SITC 2025 are as follows:
Poster 1
Title: Novel Bispecific Macrophage Engager (BiME) Antibodies Activate T Cells and Induce Durable Immune Memory to Suppress Tumor Growth
Abstract No.: 1168
Session Date: November 8, 2025
Poster 2
Title: A First-in-Human Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Preliminary Antitumor Activity of the LILRB2 Inhibitor ES009 in Patients with Advanced Solid Tumors
Abstract No.:596
Session Date: November 8, 2025
Poster 3
Title: ES038, a Novel NKCE Molecule Targeting LILRB4 in AML via Dual Engagement of NKG2A and NKG2C
Abstract No.: 435
Session Date: November 7, 2025
For more information about the meeting and posters, please visit the SITC official website: https://www.sitcancer.org/2025/home
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Leading Cross-Boundary Dialogue, Decoding Cutting-Edge Trends: “International Immunology Innovation Symposium” Successfully Held in HangzhouJob descriptionJob description
On September 29, 2025, Elpiscience, in collaboration with The Westlake University School of Medicine , the Chinese Society of Molecular Immunology, and Biomedical Innovation Group(BiG), successfully hosted the Symposium of Immunology Innovations: From Discovery to Therapy in Cancer and Autoimmune Diseases at Westlake University’s Yungu Campus in Hangzhou. Anchored on the global frontiers of immunology in both academia and industry, the symposium focused on oncology and autoimmune diseases—two major therapeutic domains driving immunotherapy innovation. The event aimed to convene world-leading minds, fostering deep cross-disciplinary dialogue between academia, clinical research, and industry, to accelerate the upgrade and leap of next-generation immunotherapies and promote global innovation and collaboration.
The symposium featured 13 distinguished scientists from leading global universities, research institutes, and biotech companies—including Harvard University, the University of Washington, the University of Chicago, Westlake University, Tsinghua University, Zhejiang University, Harbin Institute of Technology, Qihan Biotech, Strand Therapeutics, and Elpiscience. Centered on three major themes—Targeting T Cells, Targeting Myeloid Cells, and Technology and Clinical Advancement—the discussions spanned cutting-edge directions such as T cell engineering, myeloid cell biology, tumor microenvironment, CRISPR-based immune target identification, dendritic and granulocytic cell function, and translational medicine strategies. This high-level dialogue, combining academic depth with industrial foresight, attracted more than 300 participants from across the scientific and biopharmaceutical communities, generating renowned figures attended and an unprecedented response!


As the initiator of the symposium, Dr. Hongtao Lu, Co-founder and Chief Scientific Officer of Elpiscience, stated:“ Elpiscience is committed to pioneering scientific exploration, with immunology as its core driving force. We continue to deepen our focus and make breakthroughs in this field. Through this symposium, we aim to build a bridge connecting academia and industry, effectively accelerating the translation of fundamental immunological research into clinical applications rapidly, and transforming scientific leadership into tangible therapeutic benefits for patients as early as possible.”

Dr. Hongtao Lu, Elpiscience
Dr. Darren Ji, Co-founder and Chief Executive Officer of Elpiscience, commented in his opening remarks:“ China’s pharmaceutical innovation is entering a new phase of rapid growth, with emerging achievements that are drawing increasing global attention. In-depth, high-level scientific exchanges serve as the bridge and engine driving continuous breakthroughs in biomedical innovation and accelerating industrial transformation. Elpiscience has strategically established a global and differentiated R&D pipeline in immunotherapy. Through extensive and deep academic dialogue, we hope to advance in sync with the world’s scientific and industrial progress.”

Dr. Darren Ji, Elpiscience
As a co-organizer of the event, member of the Chinese Academy of Sciences and Dean of The Westlake University School of Medicine, Dr. Chen Dong said: “Westlake University is dedicated to nurturing physician-scientists and advancing medical innovation. We are delighted to collaborate with Elpiscience and other partners to create this platform showcasing representative cutting-edge research in immunology to both academia and industry. We also hope to enhance global collaboration across the immunology community, jointly promote medical innovation, foster the next generation of scientific talents, and ultimately address patients’ real needs through fundamental innovation and promoting translational research.”

Dr. Chen Dong, Westlake University
In recent years, immunotherapy has evolved beyond being merely a “weapon against cancer” toward a broader paradigm of immune remodeling, forming a new, more systemic therapeutic framework. At the symposium, scientists from around the world presented the latest frontiers and future trends of the field, supported by first-hand data and forward-looking perspectives.
Breaking Traditional Boundaries: New Frontiers in T Cell Immunology
Globally, T cell–based therapies have shown rapid advancement and achieved many phased results, demonstrating remarkable efficacy across multiple cancer types. However, new technologies and strategies are urgently needed to overcome the translational and clinical challenges that remain. In the symposium’s first session, leading scientists shared their latest research breakthroughs on this rapidly evolving front.
Dr. Arlene Sharpe, Chair of the Department of Immunology at Harvard Medical School and member of the U.S. National Academy of Sciences and National Academy of Medicine, shared insights into the molecular mechanisms of PD-1 blockade and introduced the latest research utilizing in vivo CRISPR platforms to identify key genes regulating T cell function. Her studies revealed that PTPN2 and the E3 ubiquitin ligase STUB1 act as novel negative regulators of CD8⁺ T cells within the tumor microenvironment, suggesting they may serve as promising new therapeutic targets. Inhibitors of PTPN2 and STUB1 could thus represent a new generation of effective cancer immunotherapy strategies.

Dr. Arlene Sharpe, Harvard Medical School
Dr. Philip D.Greenberg, Professor at the University of Washington and Head of the Immunology Program at the Fred Hutchinson Cancer Center, presented his team’s pioneering work on engineering CD8⁺ and CD4⁺ T cells to achieve durable tumor suppression. His findings demonstrated that the TGF-β signaling pathway has an important anti-tumor effect on T cells and showed that intratumoral immune suppression can be reversed through modulation, thereby enhancing therapeutic efficacy. Additionally, his team engineered FAS-41BB–enhanced T cells and attempted to simultaneously modify CD4 T cells and CD8 T cells to reverse T cell exhaustion, thereby promoting the long-acting anti-cancer effect of T cell therapy.

Dr. Philip D. Greenberg, University of Washington
Dr. Thomas Gajewski, Professor in the Departments of Pathology and Medicine at the University of Chicago and Leader of the Immunology and Cancer Program at the University of Chicago Comprehensive Cancer Center, explored new therapeutic targets in the tumor microenvironment. He highlighted the urgent need for novel strategies against “non–T cell–inflamed” tumors. Using RNA-seq analyses in animal models, his team successfully distinguished tumor-specific T cells from “bystander” T cells and identified two highly promising targets—GPR65 and KLRG1. Furthermore, PKCδ knockout technology was shown to offer a new approach to remodeling the tumor microenvironment.

Dr. Thomas Gajewski, University of Chicago
PD-1/PD-L1 inhibitors and their combination with chemotherapy have become an important therapeutic option for HCC. Dr. Chen Dong, member of the Chinese Academy of Sciences, Vice President and Dean of The Westlake University School of Medicine, has successfully identified distinct T cell subpopulations associated with clinical response and resistance through his laboratory’s systematic analysis of the dynamic changes in CD8⁺ T cell subsets in liver cancer patients before and after receiving combined immunotherapy. This study provides mechanistic insights into the underlying causes of patient responsiveness or resistance to immunotherapy and offers theoretical guidance for enhancing cytotoxic T cell function and improving therapeutic efficacy.

Dr. Chen Dong, Westlake University
Focusing on Myeloid Cells: A New Frontier in Immunotherapy
In recent years, mounting evidence has revealed the pivotal role of precisely targeting myeloid cells in immunotherapy. This area has thus emerged as a promising new frontier—often described as the next “blue ocean” of immunotherapeutic innovation. In the second session of the symposium, researchers shared their latest discoveries, demonstrating the vast potential of this direction and offering new perspectives for the field.
Dr. Ting Zhou, Researcher at Westlake University, presented his team’s work on the role of dendritic cell (DC) migration from the tumor microenvironment to lymph nodes in antitumor immunity. Through in vivo CRISPR screening, the team elucidated the mechanism by which the Pde5/cGMP signaling axis regulates DC migration and discovered that Pde5 inhibition effectively promotes DC trafficking, thereby enhancing antitumor immune responses and suppressing tumor growth. Based on these findings, they proposed sildenafil as a potential new immunotherapeutic strategy. This research was published in Nature in 2025.

Dr. Ting Zhou, Westlake University
Dr. Hongtao Lu, Co-founder and Chief Scientific Officer of Elpiscience, comprehensively introduced the latest research progress and mechanism breakthroughs of BiME® (Bispecific Macrophage Engager) platform in solid tumors. The studies demonstrated that BiME® exhibits potent and long-lasting antitumor activity in various tumor models with varying degrees of T cell infiltration, without safety side effects such as cytokine storm observed. Mechanistic analysis revealed that BiME® activates dendritic cells to specifically phagocytize tumor cells, thereby stimulating the expansion of CD8⁺ T cells within tumors and tumor clearance. Rechallenge experiments have confirmed that BiME® -induced CD8⁺ T cells possess persistent immunological memory while TCR sequencing has shown that they produce antigenic expansion effects and can even eliminate TAA-negative tumors. This achievement illustrated the "tumor immune cycle" pattern of BiME® by activating dendritic cells - CD8⁺ T cells.

Dr. Hongtao Lu, Elpiscience
Innate immune cells are emerging as a powerful force in immunotherapy. Professor Jin Zhang, Tenured Professor and “Qiushi” Distinguished Professor at Zhejiang University as well as Core Principal Investigator at the Liangzhu Laboratory, presented his team’s breakthrough in developing an engineered induced pluripotent stem cell (iPSC)-derived CAR-macrophage platform for cancer immunotherapy. The team elucidated the biological mechanism through which myeloid cells and T cells collaborate to exert multifaceted immune functions. They have also successfully pioneered the use of in vivo CAR-macrophages in multiple preclinical models of cancer and autoimmune diseases, demonstrating strong therapeutic efficacy and safety potential.

Dr. Jin Zhang, Zhejiang University
Dr. Xiaoyu Hu, Professor and Associate Dean at The Westlake University School of Medicine presented her team’s latest research on cytokine release syndrome (CRS) associated with immunotherapy. Her group uncovered the molecular mechanism of aberrant translational activation in macrophages during CRS and proposed translational regulation as a potential therapeutic target. Using RSK inhibitors (such as LJH685) as an example, pharmacological studies demonstrated that this approach effectively reduces IL-6 secretion and alleviates fever and neurotoxicity in humanized CRS mouse models—without compromising the antitumor activity of CAR-T cells.

Dr. Xiaoyu Hu, Westlake University
Dr. Laiguan Ng, Professor and Ph.D. Supervisor at The Westlake University School of Medicine, and his team identified a novel surface marker, dcTRAIL-R1, that distinguishes the T3 subset of tumor-associated neutrophils (TANs). Combined with CD101, a marker differentiating immature and mature neutrophils, the team established an innovative classification framework for TANs. Building upon fundamental neutrophil functions, they further categorized tumor-associated granulocytes into immature tumor neutrophils (ITNs), mature tumor neutrophils (MTNs), and reprogrammed tumor neutrophils (RTNs), which can be applied to various human tumors. Among these, RTNs were found to exhibit stronger pro-tumor activity, and preliminary analyses identified specific cell types that interact with RTNs within the tumor microenvironment.

Dr. Laiguan Ng, Westlake University
Integrating Cross-Disciplinary Perspectives: Mechanisms, Technologies, and Applications
Immunotherapy is currently in its golden age, with an ever-expanding array of novel targets and strategies opening new possibilities for therapeutic innovation. In the third session of the symposium, scientists explored breakthrough research findings across multiple dimensions—from fundamental mechanisms and technological methodologies to translational and clinical applications.
Dr. Jason John Luke, Chief Medical Officer of Strand Therapeutics, shared his team’s integrative multi-omics research on multiple cancer cell types, which identified a set of promising druggable targets represented by P38. He noted that the P38-MAPK pathway is hyperactivated in several malignancies, suppressing immune cell infiltration as well as key immune functions such as IFN-α/β gene expression and MHC II antigen presentation. Subsequent studies revealed that targeting P38 in combination with PD-1 inhibition significantly improves clinical outcomes in patients with PD-1–refractory tumors.

Dr. Jason John Luke, Strand Therapeutics
Dr. Yangxin Fu, Chair Professor of Oncology at Tsinghua University School of Medicine and the leading figure of the oncology discipline at Tsinghua University, introduced innovative strategies for converting “cold” tumors into “hot” tumors by reshaping the tumor microenvironment. His core approaches included: Utilizing membrane-anchored IL-12 as an mRNA vaccine adjuvant, which markedly enhances antitumor efficacy while minimizing systemic IL-12 toxicity. The addition of mtIL12 promotes the formation of a unique group of pre-effector T cells; Employing a tumor-targeted peptide–IL-2 prodrug (cis-NGR2-ProIL2) that synergizes with anti-PD-L1 antibodies in cold tumor models; Developing an anti-4-1BB–interleukin-15 fusion protein that both depletes tumor-infiltrating Treg cells and activates CD8⁺ T cells; Creating a CLDN18.2-targeted IL-2 prodrug (CLDN-ProIL2), through a dual-targeting mechanism, which enhances monoclonal antibody efficacy and ensures the safe, localized delivery of IL-2.

Dr. Yangxin Fu, Tsinghua University
Dr. Zhiwei Huang, Director of the School of Life Science and Medicine at Harbin Institute of Technology, presented his team’s discovery of a tunnel-like functional structure within the T cell receptor (TCR) that serves as a binding site for immunoregulatory molecules, directly modulating transitions among inhibitory, resting, and activated states of T cells. His team also, for the first time, structurally elucidated how γδ TCR recognizes endogenous phosphorylated antigens (PAgs) in tumor or infected cells through BTN protein complexes—providing a crucial structural foundation for the development of novel immunotherapies.

Dr. Zhiwei Huang, Harbin Institute of Technology
Dr. Luhan Yang, Co-founder and CEO of Qihan Biotech, introduced the company’s proprietary high-throughput gene-editing platform. Its representative product, QT-019B, is an “off-the-shelf” dual-target CAR-T cell therapy derived from healthy donor leukapheresis products. Through precise gene editing, the therapy targets CD19 and BCMA, while T cell receptor (TCR) expression is eliminated via gene knockout and multiplexable gene editing is employed to reduce immunogenicity—thereby minimizing NK cell- and T cell-mediated immune rejection in patients. Preclinical studies demonstrated potent efficacy, and preliminary clinical data also indicated promising safety and therapeutic potential.

Dr. Luhan Yang, Qihan Biotech
The symposium concluded with vibrant discussions and dynamic exchanges between speakers and participants, presenting a true feast of ideas that bridged fundamental immunology research and industrial innovation. Building upon its leading expertise in oncology and autoimmune research, Elpiscience continues to accelerate target discovery and clinical development. Moving forward, the company will continue to build an international high-level communication platform to promote cross-border exchanges, convey global innovative and disruptive research results, and bring more breakthrough innovative therapies to patients through more innovative insights.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Symposium of Immunology Innovations 2025Job descriptionJob description
Elpiscience will co-host the "Symposium of Immunology Innovations: From Discovery to Therapy in Cancer and Autoimmune Diseases" on September 29, 2025, together with the School of Medicine at Westlake University, the Molecular Immunology Subcommittee of the Chinese Society of Biochemistry and Molecular Biology, and the BiG Bio-Innovation Society. The event will take place at Westlake University, Hangzhou.
The symposium will focus on cutting-edge immunotherapy in cancer and autoimmune diseases, bringing together leading experts from academia and industry worldwide to discuss the latest advances from basic science to clinical application. Elpiscience is committed to advancing immunotherapy by bridging scientific innovation and industry practice.
Conference Information:



Please scan the QR code below to register. Attendance is subject to review, and a confirmation message will be sent upon approval.
 If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
VEGF/DLL4 Bispecific Antibody Meets Primary Endpoint in Phase 2/3 Clinical Trial for Biliary Tract CancerJob descriptionJob description
The VEGF/DLL4 bispecific antibody (CTX-009/ES104) has successfully achieved its primary endpoint in a Phase 2/3 clinical trial for biliary tract cancer (BTC), showing potential as a new global standard for second-line BTC treatment.On April 1, 2025, Compass Therapeutics announced promising Phase 2/3 data from their VEGF/DLL4 bispecific antibody Tovecimig (CTX-009) in second-line BTC treatment. The trial demonstrated that Tovecimig combined with paclitaxel significantly improved the objective response rate (ORR) to 17.1%, compared to just 5.3% with paclitaxel monotherapy, meeting its primary clinical endpoint. These compelling results position the Tovecimig combination as a potential new standard of care for second-line BTC treatment worldwide, bringing renewed hope to patients.
Elpiscience holds the Greater China rights to the VEGF/DLL4 bispecific antibody Tovecimig, ES104.
Key data from the trial, which enrolled 168 BTC patients randomized at a 2:1 ratio into either the Tovecimig plus paclitaxel group (n=111) or the paclitaxel-only group (n=57), include:
- An ORR of 17.1% in the Tovecimig plus paclitaxel group, including one complete response (CR), versus 5.3% in the paclitaxel-only control.
- Statistically significant ORR improvement (p=0.031) as confirmed by blinded independent central radiology review.
- Disease progression rate notably lower in the Tovecimig combination group at 16.2%, compared with 42.1% in the control group, underscoring the efficacy of Tovecimig in controlling disease progression.
- Tovecimig demonstrated favorable tolerability and safety, consistent with prior studies.
Secondary clinical endpoints, including progression-free survival (PFS) and overall survival (OS), are anticipated to be released in the fourth quarter of 2025.
Significant Market Potential in Greater China for ES104 Amid High BTC Incidence.
BTC, a highly aggressive gastrointestinal malignancy, has an incidence rate of approximately 3 per 100,000 worldwide, and this rate continues to rise. Treatment options remain limited, especially given the higher incidence rates observed in China and East Asia compared to Western regions, creating an urgent demand for innovative therapeutic solutions. Key statistics include:
- Annual new BTC cases in China estimated at approximately 55,700, significantly higher than in Western countries.
- Due to challenges in early diagnosis, most patients are diagnosed at advanced stages, resulting in about 85% progressing to second-line treatment after first-line therapy, underscoring the critical need for new, effective treatments.
- Currently limited standard-of-care options for second-line BTC treatment in China highlight the transformative potential of Tovecimig, promising superior therapeutic choices for patients and clinicians.
Elpiscience holds the rights to CTX-009/ES104 in Greater China and plans to leverage the latest clinical data to aggressively advance its development and commercialization in the region, ensuring timely access to cutting-edge treatments for local patients. Meanwhile, Compass Therapeutics continues to explore the potential of Tovecimig in other prevalent cancers, such as colorectal cancer, aiming to expand indications and bring hope to an even broader population of cancer patients.
[1]Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022;2:1-9.8
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience will be hosting International Symposium on Cell Engagers and Cancer ImmunotherapyJob descriptionJob description
We are proud to announce that Elpiscience will be hosting the prestigious International Symposium on Cell Engagers and Cancer Immunotherapy in Crowne Plaza, Suzhou, China on June 28-29, 2024.
This highly anticipated event will bring together leading experts from academia and industry to discuss the cutting-edge advancements and future potential of developing cell engagers into game-changing options for immunotherapies.
With a lineup of world-class speakers and the participation of over 100 industry experts, this symposium promises to be a truly enlightening experience. Attendees will have the opportunity to engage in thought-provoking discussions and gain valuable insights into the latest innovations in the field of cell engagers and immuno-oncology.
Learn more about some of our esteemed speakers and the exciting topics they will be presenting. To apply for attendance, please scan the QR code in the flyer or via below link. A confirmation will be sent to you after the application is approved.
https://www.wjx.cn/vm/mpTPdQP.aspx If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces the Appointment of Jason John Luke, M.D., to its Scientific Advisory BoardJob descriptionJob description
Shanghai, Suzhou, China April 3,2024 Elpiscience Biopharmaceuticals announces the Appointment of Jason Luke M.D., to its Scientific Advisory Board.
“We are excited to have Dr. Luke join our SAB,” said Dr.Hongtao Lu Co-Founder and Chief Scientific Officer of Elpiscience, “Dr. Luke’s expertise in clinical and translational immunotherapy as well as his experience in early-stage drug development for solid tumors complement our existing group of renowned scientific experts.”
Dr. Luke is an Associate Professor of Medicine at the University of Pittsburgh and Director of the Immunotherapy and Drug Development Center as well as the Associate Director for Clinical Research at the UPMC Hillman Cancer Center. Previously he was an Assistant Professor of Medicine at the University of Chicago, an Attending Physician at Brigham and Women’s Hospital in Boston, and an Instructor in Medicine at Harward Medical School.
Dr. Luke earned his Doctor of Medicine from the Rosalind Franklin University of Medicine and Science/Chicago Medical School and obtained a Bachelor's degree in Microbiology from the University of Lowa. He completed his training as a resident at Boston University Medical Center. Dr. Luke undertook the fellowship of medicine at Weill Cornell Medical Center, New York, and the fellowship of Medical Oncology at Memorial Sloan-Kettering Cancer Center, New York.
About Elpiscience’s Scientific Advisory Board
Dr. Thomas Gajewski from the University of Chicago
Dr. Philip D. Greenberg from the University of Washington
Dr. Jason John Luke from the University of Pittsburgh
Dr. Vijay Kuchroo from Havard Medical School
Dr. Arlene Sharpe from Harvard Medical School
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience and Astellas Enter into Research Collaboration and License Agreement for Novel Bispecific Macrophage EngagerJob descriptionJob description
Shanghai and Suzhou, TOKYO, December 28, 2023 - Elpiscience Biopharma, Ltd. (Chairman and CEO: Darren Ji, MD, Ph.D., “Elpiscience”) and Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, “Astellas”) today announced a research collaboration and license agreement for novel bi-specific macrophage engagers, ES019 and another program. The two companies will collaboratively conduct early-stage research for these two programs. Elpiscience will also grant Astellas the right to add up to two additional programs to be included in the collaboration. If Astellas exercises its option, Elpiscience will grant Astellas the exclusive right to further research, develop, manufacture and commercialize the products for each program.
Elpiscience is a privately held, clinical-stage biopharmaceutical company dedicated to developing next-generation immuno-oncology therapies for cancer patients worldwide. Their Bispecific Macrophage Engager Platform (BiME®) is anti-tumor associated antigen (TAA) and anti-signal-regulatory protein α (SIRPα) bispecific antibody-based platform to activate Tumor Associated Macrophage (TAM) phagocytosis killing towards specific TAA expressing tumor cells. BiME® shows highly potent phagocytosis due to engagement of the Fc receptor on TAM and the tumor cells via TAA and SIRPα, and blockade of CD47-SIRPα “don’t eat me” signaling1. This platform is utilized for ES019, an anti-PD-L1/SIRPα bispecific antibody.
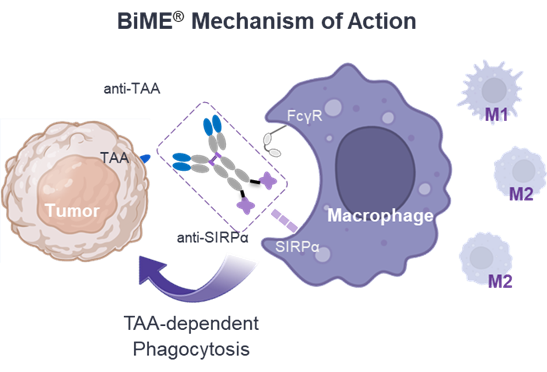
TAMs are the most abundant leukocytes within Tumor Microenvironment (TME) of many cancer types and correlate with poor prognosis and immune checkpoint inhibitor resistance. The programs emerging from the BiME® platform are expected to offer new options for cancer patients who do not respond to existing cancer immunotherapies, by modulating TAM and reprogramming the TME status.
Elpiscience will receive up to US $37 million, including the upfront payment and license option fees. In addition, Elpiscience will receive research funding from Astellas to advance the programs. After Astellas exercises its option, Elpiscience is eligible to potentially receive more than US $1.7 billion in payments for the achievement of future development, regulatory, and commercial milestones. Elpiscience is also eligible to receive single-digit to lower double-digit percent royalty payments on net sales for licensed products per each program.
Darren Ji, MD, Ph.D., Chairman and CEO, Elpiscience
“We are pleased to collaborate with Astellas, a world leader of innovative medicines, on developing game-changing therapies for cancer. BiME® innovated at Elpiscience represents a paradigm shift from the conventional cell engagers dominated by T cells. The therapeutic molecules generated from the BiME® platform have the potential of changing the clinical practice for tumors where tumor-associated macrophages are highly abundant, and no effective therapies are available. We look forward to working with Astellas to bring these exciting sciences to the bedside of global patients.Adam Pearson, Chief Strategy Officer, Astellas
“Astellas has a strong commitment to developing innovative cancer treatments and have positioned Immuno-Oncology as one of the Primary Focuses of our R&D strategy2. Elpiscience has outstanding expertise in developing next generation immunotherapies. We hope this collaboration will bring synergies between the two companies' cutting-edge research and will ultimately lead to the development of new treatments for patients with cancer.”1 “don’t eat me” signaling: CD47 is a protein that emits a "don't eat me" signal. When CD47 on tumor cells bind with SIRPα on macrophages, the macrophages suppress phagocytosis.
2 Astellas’ R&D strategy: Astellas has established a Focus Area Approach for its research and development strategy. For more information, please visit our website at Areas of Interest | Astellas Pharma Inc.About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company dedicated to developing life-changing immuno-oncology therapies for cancer patients worldwide. The company’s innovative approach is focused on targeting tumor microenvironment to identify effective mechanisms to kill cancer. A pipeline of novel molecules has been developed using its proprietary platforms including a powerful Bispecific Macrophage Engager (BiME®) technology that connects and activates macrophages for solid tumor killing without causing cytokine storms. For more information, please visit: www.elpiscience.com.About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into VALUE for patients. For more information, please visit our website at https://www.astellas.com/en.Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management’s current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas’ intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.Contacts for inquiries or additional information:
Elpiscience Biopharma, Ltd.
PR@elpiscience.com
Astellas Pharma Inc.
Corporate Communications
+81-3-3244-3201If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Studies Presented at Society for Immunotherapy of Cancer (SITC) 2023 Annual MeetingJob descriptionJob description
Elpiscience Biopharmaceuticals presented studies for its innovative immunotherapeutic molecules at the SITC 2023 Annual Meeting, including KG2A/NKG2C dual-targeting antibody ES015-2, a high affinity LILRB1 specific blocking antibody ES008-a, and the first-in-class anti-CD39/TGF-βRII bifunctional fusion protein ES014.
Study Highlights:
Title: Selective delivery of TGFβ “trap” to CD39-expressing immune and stroma cells reshapes tumor microenvironment and rejuvenates antitumor immunity
Abstract Number: 453CD39-Adenosine and TGFβ are two key immune suppressive pathways within the tumor microenvironment (TME). TGFβ, in contrast to its biphasic effects on tumor cells, acts on stromal cells and immune cells in the TME, which commonly express high levels of CD39, to promote tumor progression. CD39-targeted TGFβ “trap” is thus more likely to effectively inhibit tumor progression. Our study showed that ES014, a bifunctional antibody–ligand trap which comprises an antibody targeting CD39 fused to a TGFβ receptor II ectodomain, can inhibit TGFβ activity and lead to cancer killing in ex vivo models.
A phase I clinical study is ongoing to primarily investigate the safety, tolerability, and preliminary clinical activity of ES014 in patients with advanced solid tumors.
Highlights:
- ES014 binds and neutralizes both CD39 and TGFβ.
- ES014 promoted killing of tumors from NSCLC patients in ex vivo MPE model.
- ES014 inhibits Treg differentiation and TGFβ-induced CD39 expression on T cells.
- ES014 promotes T cell survival.
Title: ES015-2, a first-in-class NKG2A and NKG2C dual-targeting antibody, demonstrated potent anti-tumor immune response
Abstract Number: 498The inhibitory receptor NKG2A and the activating receptor NKG2C modulate the function of NK and CD8 T cells by recognizing the same ligand HLA-E. NKG2A is selectively expressed on lymphocytes with cytolytic function, and the NKG2C “engager” has the potential to generate a strong antitumor response against various tumors. Inhibiting NKG2A and HLA-E alone was not as effective as had been expected in both mouse tumor models and in clinical trials. Our NKG2A/NKG2C dual targeting antibody ES015-2 can inhibit NKG2A function yet promoting NKG2C action, leading to superior anti-tumor response.
Highlights:
- ES015-2 is a NKG2A/NKG2C dual targeting antibody.
- ES015-2 can completely block the interaction of NKG2A/CD94 with HLA-E, thereby inhibits HLA-E-induced NKG2A inhibitory signaling.
- Ligation of ES015-2 effectively potentiates the activation of NKG2C+ NK cells and T cells.
Title: ES008-a, a high affinity LILRB1 specific blocking antibody activates multiple immune cells to fight cancers
Abstract Number: 510LILRB1 is the most broadly expressed member of LILRB family on various immune cells. Blocking LILRB1 augments macrophage phagocytosis of tumor cells, restores cytotoxic function of NK cells, and enhances tumor cell killing by effector CD8+ T cells. The high affinity LILRB1 specific blocking antibody ES008-a can activate multiple immune cells to fight cancers.
Highlights:- ES008-a is a high affinity LILRB1-specific blocker that can completely block HLA-G/LILRB1 and HLA-A2/LILRB1 interactions.
- ES008-a promotes NK cell-mediated destruction of tumor cells.
- ES008-a synergizes with CD47/SIRPα inhibitors in enhancing macrophage phagocytosis of tumor cells.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Three Poster Presentations at Society for Immunotherapy of Cancer (SITC) 2023 Annual MeetingJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company focused on developing next-generation immunotherapies to benefit cancer patients worldwide, today announced it will have three poster presentations at the SITC 2023 Annual Meeting taking place November 3-5, 2023, in San Diego. The posters will highlight its studies on three innovative molecules including a first-in-class NKG2A/NKG2C dual-targeting antibody ES015, a high affinity LILRB1 specific blocking antibody ES008-a, and the first-in-class anti-CD39/TGF-βRII bifunctional fusion protein ES014 which has been proven to be able to deliver TGFβ “trap” to CD39-expressing immune and stroma cells to reshape tumor microenvironment and rejuvenates antitumor immunity.
Poster presentation details:
Title: Selective delivery of TGFβ “trap” to CD39-expressing immune and stroma cells reshapes tumor microenvironment and rejuvenates antitumor immunity
Abstract Number: 453
Date and time: November 3, 2023; 9 am –7 pm PDT
Location: Exhibit Halls A and B1 – San Diego Convention CenterTitle: ES015, a first-in-class NKG2A and NKG2C dual-targeting antibody, demonstrated potent anti-tumor immune response
Abstract Number: 498
Date and time: November 4, 2023; 9 am –8:30 pm PDT
Location: Exhibit Halls A and B1 – San Diego Convention CenterTitle: ES008-a, a high affinity LILRB1 specific blocking antibody activates multiple immune cells to fight cancers
Abstract Number: 510
Date and time: November 4, 2023; 9 am –8:30 pm PDT
Location: Exhibit Halls A and B1 – San Diego Convention CenterIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces First Patient Dosed for Phase 1 Clinical Trial of Anti-LILRB2 Antibody ES009 in AustraliaJob descriptionJob description
Elpiscience Biopharmaceuticals (“Elpiscience”), a clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, announced today that the first patient has been dosed in a Phase 1 clinical trial of its anti-LILRB2 monoclonal antibody ES009 in Australia. The objective of the trial is to evaluate its safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary clinical activity.
LILRB2, also known as ILT4, is an inhibitory receptor widely expressed on the surface of myeloid cells that also contributes significantly to immune suppression in the tumor microenvironment (TME). ES009 specifically binds to a unique epitope on human LILRB2 and potently blocks LILRB2 binding to multiple ligands. By blocking LILRB2-mediated inhibitory signaling, ES009 can reprogram myeloid cells from anti-inflammatory phenotype into pro-inflammatory phenotype, and reinvigorate T cell functionalities. ES009 has demonstrated exceptional potential to reverse immune suppression in the TME and promote anti-tumor immunity in preclinical studies.LILRB2 is a key immune checkpoint for tumor immunotherapy whose suppression of immune response is proved as one mechanism of anti-PD(L)1 resistance. With a keen interest in revolutionizing therapies for cancer patients non-responsive or resistant to PD(L)1 treatment, we developed ES009 which has shown best-in-class potential. We believe in the power of enhanced innate immunity in treating cancer and have developed a highly differentiated myeloid cell focused therapeutics portfolio. We will work relentlessly to bring these highly promising therapies to cancer patients with unmet medical needs," said Dr. Hongtao Lu, Co-founder and CSO of Elpiscience.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present at BIO-Europe Spring 2023 in BaselJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company dedicated to developing life-changing immuno-oncology therapies for cancer patients worldwide, today announced that it will be presenting in person at the 17th annual BIO-Europe Spring taking place March 20-22, 2023 in Basel, Switzerland.
Elpiscience has been focused on removing immunosuppressive factors in the tumor microenvironment, by targeting the adenosine pathway and myeloid checkpoints. Darren Ji, Co-founder and CEO of Elpiscience, will present the company’s core pipeline programs including its Phase 1 ES014, a first-in-class anti-CD39/TGF-β bispecific antibody that is expected of having single agent anti-tumor activity, a highly differentiated anti-LILRB2 antibody ES009 that effectively reprogram macrophage from M2 to M1, and a pan-acting anti-SIPRα antibody ES004 with a larger population coverage.
Darren Ji will also join a panel to discuss the involving APAC partnering paradigms on March 21,2023 during the event.
Presentation schedule:- Date and Time: March 20th, 13:00-13:15 (CET)
- Presenter: Darren Ji, Co-Founder and CEO of Elpiscience
- Venue: Room 2, Messe Basel, Basel, Switzerland
Panel schedule:
- Panel Discussion: APAC partnering: where are we and where does the future lie?
- Panelist: Darren Ji, Co-Founder and CEO of Elpiscience
- Date and Time: March 21st, 10:30 - 11:15 (CET)
- Venue: Room 1, Messe Basel, Basel, Switzerland
About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company dedicated to developing life-changing immuno-oncology therapies for cancer patients worldwide. The company’s innovative approach is focused on removing immunosuppressive factors in the tumor microenvironment, by targeting the adenosine pathway and myeloid checkpoints. A pipeline of novel molecules has been developed using its proprietary platforms including a powerful Bispecific Macrophage Engager (BiME®) technology that connects and activates macrophages for solid tumor killing without causing cytokine storms.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces First Patient Dosed for Phase 1 Clinical Trial of First-in-Class Anti-CD39xTGF-β Bispecific Antibody ES014Job descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company dedicated to developing life-changing immuno-oncology therapies for cancer patients worldwide, today announced that the first patient has been dosed in a Phase 1 clinical trial of ES014, a first-in-class anti-CD39xTGF-β bispecific antibody (bsAb). The principal investigator of the study is Professor Lu Shun, Director of Oncology Department of Shanghai Chest Hospital.
ES014 simultaneously targets the CD39-adenosine and TGF-β pathways, aiming to convert the immunosuppressive tumor microenvironment (TME) into an immune-friendly one. Adenosine and TGF-β are two key immunosuppressive pathways within the TME. TGF-β, frequently expressed on solid tumors, suppresses T cell activation and induces CD39 expression, the rate-limiting enzyme in the ATP-adenosine pathway. ES014 is designed to have the following functions: 1. It blocks suppressive adenosine generation through CD39 inhibition while maintaining high levels of immune-stimulatory extracellular ATP, the substrate of CD39; 2. ES014 simultaniously neutralizes TGF-β, leading to activation of T cells and blockade of Treg differentiation while avoiding or minimizing systemic immunotoxicity.

Dr. Steve Chin, Chief Medical Officer of Elpiscience
“We are excited to have initiated the trial of this novel drug candidate. I’m proud of our highly committed team for their exceptional execution of the project. ES014 has shown strong single-agent anti-tumor efficacy in PD-1 non-responsive animal model. We look forward to seeing positive impact in cancer patients, ” said Dr. Steve Chin, Chief Medical Officer of Elpiscience.

Professor Lu Shun, Director of Oncology Department of Shanghai Chest Hospital
“I’m glad to lead the clinical study of ES014 in China. We have observed significant immune-promoting and tumor killing effect by ES014 in our ex vivo model of pleural effusion and ascites samples from patients with cancer. I am excited to move ES014 now to clinical study to investigate its safety and preliminary clinical signal in human.” shared Professor Lu.
About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company dedicated to developing life-changing immuno-oncology therapies for cancer patients worldwide. The company’s innovative approach is focused on removing immunosuppressive factors in the tumor microenvironment, by targeting the adenosine pathway and myeloid checkpoints. A pipeline of novel molecules has been developed using its proprietary platforms including a powerful Bispecific Macrophage Engager (BiME®) technology that connects and activates macrophages for solid tumor killing without causing cytokine storms.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present Its Novel Immunotherapies at Biotech Showcase™ during J.P. Morgan Week 2023Job descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, today announced that it will present the latest progress of its innovative molecules and powerful antibody generation platforms at the Biotech Showcase™ conference during J.P.Morgan Week 2023 in San Francisco in January. Elpiscience’s Co-Founder and CEO Darren Ji will also be sharing his perspective on the BioPharma Business in the Asia Pacific Region: Huge Markets, Great Opportunities panel at the conference.
“We are excited to bring to the world our novel immunotherapies, including our first-in-class anti-CD39/TGF-β bispecific antibody ES014 potentially for PD-(L)1 resistant patient population, a highly differentiated anti-LILRB2 antibody ES009, and a pan-acting anti-SIPRα antibody ES004. We look forward to collaborating with pharmaceutical partners to advance our innovative drug candidates to benefit cancer patients worldwide,” said Darren Ji, Co-Founder, and CEO of Elpiscience.
Event Schedules:Elpiscience’s presentation
Darren Ji, Co-Founder and CEO of Elpiscience
Date and Time: 9 January 2023, 14:30 – 14:45 PST
Location: Hilton Union Square, Franciscan C (Ballroom Level), San Francisco CAPanel: BioPharma Business in the Asia Pacific Region: Huge Markets, Great Opportunities
Darren Ji, Co-Founder and CEO of Elpiscience
Date and Time: 10 January 2023, 9:00 – 10:00 PST
Location: Hilton Union Square, Imperial Ballroom, San Francisco CAElpiscience’s team is participating in partnering system and welcomes the opportunity to meet with potential partners. For meeting appointments, registered attendees can send a request through Biotech Showcase Partnering Platform, and or by contacting Elpiscience directly at BD@elpiscience.com.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces China CDE IND Clearance of ES014, a First in Class Anti-CD39xTGF-β Bispecific Antibody for Patients with Advanced Solid TumorsJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company focused on developing next-generation immunotherapies to benefit cancer patients worldwide, today announced that the China Center of Drug Evaluation (CDE) for National Medical Products Administration has cleared Elpiscience’s Investigational New Drug Application (IND) for ES014 to initiate a Phase 1 clinical study for patients with advanced solid tumors. ES014 is a first-in-class anti-CD39xTGF-β bispecific antibody (bsAb) that simultaneously targets the CD39-adenosine and TGF-β pathways to synergistically activates T cells for ICB-resistant cancer immunotherapy.
“We are delighted that our IND for ES014 was cleared by CDE. Solid tumors frequently express TGF-β, which suppresses T cell activation and induces CD39 expression, the rate-limiting enzyme in the ATP-adenosine pathway. The anti-CD39 target is designed to selectively direct ES014 to the TME where CD39 expression level is high and the anti-TGF-β activity promotes effector T cell entry into TME, resulting in immune activation and eventually tumor killing, while avoiding or minimizing systemic immunotoxicity,” said Dr. Hongtao Lu, Co-Founder and Chief Scientific Officer of Elpiscience.
ES014’s anti-CD39 activity aims to reverse TME immunosuppression by reducing suppressive adenosine, while maintaining high levels of immune-stimulatory extracellular ATP. The combined removal of immune suppression and immune stimulating effects of ES014 were recently demonstrated in a PD-1 antibody non-responsive in vivo animal model where tumor growth was significantly inhibited after treatment.
“ES014 is an innovative product targeting suppressive tumor microenvironment, aiming to convert ‘cold’ tumor into ‘hot’ tumor by simultaneously blocking ATP-adenosine and TGF-beta pathways, the two most important suppressive pathways within TME. In ex vivo human cancer 3D studies, ES014 has demonstrated excellent ability in promoting CD8 T cell survival and T cell cytotoxicity towards cancer. We look forward to seeing cancer patients deriving benefit in our Phase 1 study,” said Dr. Steve Chin, Chief Medical Officer of Elpiscience.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Studies Presented at SITC 2022 Annual MeetingJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company focused on developing next-generation immunotherapies to benefit cancer patients worldwide, presented positive studies for its innovative immunotherapeutic molecules at the SITC 2022 Annual Meeting, including anti-SIRPα monoclonal antibody ES004, PD-L1/SIRPα bispecific antibody ES019, anti-LILRB2 monoclonal antibody ES009, anti-SIGLEC15 antibody ES012, and anti-LAG3 monoclonal antibody ES005,.
Study highlights:1. Title: Treatment of anti-SIRPα in combination with anti-TAA exerts superior anti-tumor activity
Abstract No.: 793SIRPα is an inhibitory receptor expressed mainly on myeloid cells and dendritic cells. Ligation of CD47 to SIRPα delivers a “don’t eat me” signal to suppress macrophage phagocytosis. Tumor cells frequently overexpress CD47 to evade macrophage-mediated destruction. Anti-SIRPα mAb ES004 potently potentiates antibody dependent cellular phagocytosis (ADCP) activity of antibodies against tumor associated antigens (TAAs) in vitro and in vivo.
Highlights:
• ES004 recognizes pan-allele human SIRPα with high affinity
• ES004 binds to a unique epitope on the CD47 binding domain of SIRPα
• ES004 potently blocks CD47-SIRPα interaction and CD47 induced SIRPα signaling
• ES004 effectively potentiates pan-allelic macrophage phagocytosis of tumor cells
• ES004 has no negative impact on T cell activation
• ES004 enhances anti-tumor activity in combination with anti-Claudin18.2 in MC38/hCLDN18.2 syngenetic tumor model
• ES004 has demonstrated favorable PK, full target occupancy and excellent safety profile in cynomolgus monkeys.
2. Title: Dual targeting of innate and adaptive immune checkpoints with a PD-L1/SIRPα bispecific macrophage engager to promote anti-tumor activity
Abstract No.: 1211The anti-PDL1/SIRPα bispecific antibody ES019, designed for tumor cell and immune cell dual targeting, is capable of reactivating macrophages and T cells to kill cancer cells with the potential to overcome the limitations of traditional anti-PD1 therapies and has demonstrated significantly enhanced tumor therapeutic efficacy and specificity versus combo or monotherapies.
Highlights:
• ES019 phagocytosis activity is corrected with PD-L1 level on tumor cells
• ES019 leads to better phagocytosis capability of tumor cells by M2-like than M1-like macrophage
• ES019 activates T cells without induction of phagocytosis of T cells
• ES019 shows favorable PK in mouse model
• ES019 demonstrates single agent anti-tumor efficacy in animal model
3. Title: ES009, a LILRB2-specific blocking antibody, reprograms myeloid cells into pro-inflammation phenotype and potentiates T cell activation
Abstract No.: 1062LILRB2 is predominantly expressed in myeloid lineage cells. Human LILRB2 broadly binds to multiple ligands and contributes to immune suppression in the tumor microenvironment (TME). Anti-LILRB2 mAb ES009 has demonstrated superior effects in converting immunosuppressive myeloid cells into pro-inflammation phenotypes in in vitro and ex vivo models.
Highlights:
• ES009 specifically binds human LILRB2 with high affinity
• ES009 binds to a unique epitope on D1 domain of LILRB2
• ES009 potently blocks LILRB2 binding to multiple ligands
• ES009 promotes monocytes and monocytes derived DCs into a pro-inflammatory status
• ES009 effectively reprograms human monocyte derived M2 macrophages into pro-inflammation M1 phenotypes
• ES009 effectively relives T cells from M2 macrophages mediated suppression
• ES009 converts primary macrophages in malignant ascites in ovarian cancer patients into a pro-inflammatory status
4. Title: SIGLEC15 induces monocyte apoptosis and an SIGLEC15 antibody ES012 reverses myeloid cells driven immunosuppression
Abstract No.: 1401SIGLEC15 is a glycan-recognition proteins belonging to the SIGLEC family and is highly expressed on TAM and many tumor cells. It’s reported that SIGLEC15 inhibits T cell activity via its binding to an unknown receptor on T cells. Elpiscience has identified a novel function of SIGLEC15 that SIGLEC15 can induce monocyte apoptosis and its inhibitory effect on T cell function is indirect. Based on this newly discovered SIGLEC15 biology, we have developed a potent, functional anti-SIGLEC15 mAb ES012 that has great potential to reverse immune suppression in TME to promote anti-tumor immunity.
Highlights:
• SIGLEC15 induces monocyte apoptosis, which is dependent on sialic acid binding and mediated via caspase-3
• SIGLEC15 inhibits T cell function via myeloid cells but not by directly binding to T cells
• ES012 is a high affinity anti-SIGLEC15 monoclonal antibody.
• ES012 can rescue monocyte apoptosis and inhibit T cells by SIGLEC15.
• ES012 showed superior anti-tumor efficacy and better PK profile than benchmark antibody in preclinical model
5. Title: ES005, a high affinity anti-LAG3 monoclonal antibody, inhibits the interactions between LAG3 and multiple ligands and enhances anti-tumor activity of T cells in preclinical models
Abstract No.: 426LAG3 plays an important role in regulating immune homeostasis with multiple biological activities related to T cell functions. Anti-LAG3 mAb ES005 has demonstrated significant tumor growth inhibition in in vivo mouse tumor models, and showed excellent PK and safety profile in NHPs, indicating great potential to be used as next-generation immune checkpoint inhibitor in cancer treatment.
Highlights:
• ES005 is a high affinity and cynomolgus reactive anti-LAG3 mAb
• ES005 binds to a unique epitope on LAG3 D1 domain
• ES005 potently blocks LAG3 binding to multiple ligands including FGL-1
• ES005 effectively upregulates NFAT reporter gene transcription via blocking LAG3/MHC-II interaction
• ES005 effectively reverses LAG3 driven inhibition of T cell activation
• ES005 monotherapy potently inhibits tumor growth in EMT6 syngeneic tumor model
• ES005 was well tolerated in cynomolgus monkeys with favorable PK Profile
Download full posters:
ES004: Treatment of anti-SIRPα in combination with anti-TAA exerts superior anti-tumor activity
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Five Poster Presentations at Society for Immunotherapy of Cancer (SITC) 2022 Annual MeetingJob descriptionJob description
SHANGHAI, China and SUZHOU, China and GERMANTOWN, MD., Oct. 20, 2022 – Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company focused on developing next-generation immunotherapies to benefit cancer patients worldwide, today announced it will have five poster presentations at the SITC 2022 Annual Meeting being held in Boston, Massachusetts from November 8-12. The posters will highlight preclinical studies on five innovative molecules including anti-SIRPα monoclonal antibody ES004, anti-LAG3 monoclonal antibody ES005, anti-LILRB2 monoclonal antibody ES009, anti-SIGLEC15 antibody ES012, and PD-L1/SIRPα bispecific macrophage engager ES019.
Poster presentations:
1.
Title: ES005, a high affinity anti-LAG3 monoclonal antibody, inhibits the interactions between LAG3 and multiple ligands and enhances anti-tumor activity of T cells in preclinical models
Abstract No.: 426
Date and time: 11/11/2022, 9:00 am - 9:00 pm (EST)
2.
Title: ES009, a LILRB2-specific blocking antibody, reprograms myeloid cells into pro-inflammation phenotype and potentiates T cell activation
Abstract No.: 1062
Date and time: 11/11/2022, 9:00 am - 9:00 pm (EST)
3.
Title: Treatment of anti-SIRPα in combination with anti-TAA exerts superior anti-tumor activity
Abstract No.: 793
Date and time: 11/10/2022, 9:00 am - 9:00 pm (EST)
4.
Title: Dual targeting of innate and adaptive immune checkpoints with a PD-L1/SIRPα bispecific macrophage engager to promote anti-tumor activity
Abstract No.: 1211
Date and time: 11/10/2022, 9:00 am - 9:00 pm (EST)
5.
Title: SIGLEC15 induces monocyte apoptosis and an SIGLEC15 antibody ES012 reverses myeloid cells driven immunosuppression
Abstract No.: 1401
Date and time: 11/10/2022, 9:00 am - 9:00 pm (EST)
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces FDA IND Clearance of ES014, a First in Class Anti-CD39xTGF-β Bispecific Antibody for Patients with Advanced Solid TumorsJob descriptionJob description
SHANGHAI & SUZHOU, China & GERMANTOWN, Md., May 9, 2022- Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company focused on developing next-generation immunotherapies to benefit cancer patients worldwide, today announced that the U.S. Food and Drug Administration (FDA) has cleared Elpiscience’s ES014 Investigational New Drug (IND) Application to initiate a Phase 1 clinical study for patients with advanced solid tumors. ES014 is a first in class anti-CD39xTGF-β bispecific antibody (bsAb) that simultaneously targets the CD39-adenosine and TGF-β pathways, two major immunosuppressive mechanisms in the tumor microenvironment (TME).
“We are delighted that our IND for ES014 was cleared by FDA,” said Steve Chin, Chief Medical Officer of Elpiscience. “ES014 is a first-in-class anti-CD39xTGF-β bispecific antibody. In preclinical studies, ES014 demonstrated significant anti-tumor activity in a PD-1 resistant in vivo efficacy model. We look forward to starting the Phase 1 study soon.”
Solid tumors frequently express TGF-β, which suppresses T cell activation and induces CD39 expression, the rate-limiting enzyme in the ATP-adenosine pathway. The anti-CD39 target is designed to selectively direct ES014 to the TME where the anti-TGF-β activity promotes effector T cell function and immune activation, while avoiding or minimizing systemic immunotoxicity. ES014’s anti-CD39 activity further aims to reverse TME immunosuppression by reducing suppressive adenosine, while maintaining high levels of immune-stimulatory extracellular ATP. The combined removal of immune suppression and immune stimulating effects of ES014 were recently demonstrated in a PD-1 antibody non-responsive in vivo animal model where tumor growth was significantly inhibited after treatment.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Positive Interim ES104 Phase 2 Data Reported in Combination with Paclitaxel in Biliary Tract CancersJob descriptionJob description
SHANGHAI & SUZHOU, China & GERMANTOWN, Md., May 6, 2022- Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a clinical-stage biopharmaceutical company focused on developing next-generation immunotherapies to benefit cancer patients worldwide, today announced partner Compass Therapeutics reported positive interim ES104 (also known as CTX-009) Phase 2 data in combination with paclitaxel in patients with biliary tract cancers (BTC). ES104 is a bispecific antibody that simultaneously blocks Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways, which are critical to angiogenesis and tumor vascularization.
In the study, ES104 demonstrated:
• 42% overall response rate (ORR) based on 10 patients with Partial Responses (PRs), including 9 PRs confirmed by RECIST 1.1 and 1 PR pending confirmation
• Anti-tumor activity in previously treated patients with a clinical benefit rate (CBR) of 92% based on 22 patients with a PR or stable disease (SD) out of 24 enrolled patients
• Well-tolerated and preliminary safety profile consistent with prior studiesThe Phase 2 study in patients with BTC is currently being conducted at four leading medical centers in Korea and Compass Therapeutics plans to open additional sites in the United States. In China, Elpiscience is currently enrolling patients in a ES104 Phase 1/2 study for the treatment of unresectable locally advanced or metastatic colorectal cancer (CRC). ES104 is currently the only clinical-stage bispecific antibody targeting VEGF and DLL4 in China.
“We are encouraged by the impressive interim Phase 2 data in BTC patients from our partner,” said Steve Chin, Chief Medical Officer of Elpiscience. “ES104 in combination with paclitaxel showed a high ORR including responses in all four BTC subtypes and good overall tolerability consistent with prior studies. We believe in the clinical strategy to simultaneously target VEGF and DLL4 and look forward to our study findings in advanced CRC patients.”
For more information on the Phase 1/2 clinical study in patients with unresectable locally advanced or metastatic CRC, refer to Clinicaltrials.gov identifier NCT05167448.
About ES104ES104 is a bispecific antibody that simultaneously blocks Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways, which are critical to angiogenesis and tumor vascularization. Pre-clinical and early clinical data of ES104 show that blocking both pathways provides robust anti-tumor activity across several solid tumors, including colorectal, gastric, cholangiocarcinoma, pancreatic, and non-small cell lung cancer. Partial responses to ES104 as monotherapy have been observed in heavily pre-treated cancer patients, who were resistant to currently approved anti-VEGF therapies. ES104 has completed a Phase 1 monotherapy dose-escalation and expansion study (NCT03292783). Phase 1b and Phase 2 clinical studies (NCT04492033) in combination with chemotherapy are ongoing. Elpiscience licensed ES104 greater China rights from Compass Therapeutics in January 2021 and is conducting Phase 1/2 study in China for treatment in patients with unresectable locally advanced or metastatic colorectal cancer.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces First Patient Dosed in Phase 1/2 Clinical Study of ES104 for Treatment of Colorectal CancerJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, today announced that the first patient has been dosed in a Phase 1/2 clinical study, evaluating the safety, tolerability, preliminary anti-tumor efficacy and pharmacokinetics of ES104 for the treatment of unresectable locally advanced or metastatic colorectal cancer (CRC) in China. ES104 is a bispecific antibody that simultaneously blocks the Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways that are critical to angiogenesis and tumor vascularization.
In prior clinical testing, ES104 demonstrated significant anti-tumor activity in patients who had failed multiple lines of therapy and were considered resistant to currently approved anti-VEGF therapies. Elpiscience received Center of Drug Evaluation (CDE) IND clearance for ES104 in October 2021. ES104 is currently the only clinical-stage bispecific antibody targeting VEGF and DLL4 in China.
“We are excited to initiate this Phase 1/2 clinical study of ES104. In a recent Phase 1 study, ES104 showed single-agent activity in advanced gastric cancer and CRC patients who were considered treatment resistant to anti-VEGF containing regimens,” said Steve Chin, CMO of Elpiscience. “We look forward to the potential therapeutic benefit of ES104 for the treatment of CRC patients in China.”
For more information on the Phase 1/2 clinical study, refer to Clinicaltrials.gov identifier NCT05167448.
About ES104:
ES104 is a bispecific antibody that simultaneously blocks Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways, which are critical to angiogenesis and tumor vascularization. Pre-clinical and early clinical data of ES104 show that blocking both pathways provides robust anti-tumor activity across several solid tumors, including colorectal, gastric, cholangiocarcinoma, pancreatic, and non-small cell lung cancer. Partial responses to ES104 as monotherapy have been observed in heavily pre-treated cancer patients, who were resistant to currently approved anti-VEGF therapies. ES104 has completed a Phase 1 monotherapy dose-escalation and expansion study (NCT03292783). Phase 1b and Phase 2 clinical studies (NCT04492033) in combination with chemotherapy are ongoing. Elpiscience licensed greater China rights to ES104 in January 2021.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Participate in Cross-Pacific Collaboration Panel at BIO CEO & Investor ConferenceJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, today announced that its co-founder and chief executive officer, Dr. Darren Ji, will participate in a panel discussion about R&D and innovation leading cross-pacific collaboration. The conference takes place at the Marriott Marquis in New York City from February 14 to 15 and virtually through February 17, 2022.
The panel, titled "What is New in Cross-Pacific Deal Making and Investment", will take place from 9:00 a.m. to 9:45 a.m. EST Wednesday, February 16, 2022, and will be accessible live to conference registrants.
A recorded Elpiscience presentation will also be available to registered attendees through the event platform beginning February 11.
For additional information, visit BIO CEO & Investor Conference.
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focused on innovating and developing next-generation immunotherapy to benefit cancer patients worldwide. The company has a robust pipeline of globally innovative molecules, covering wide range of targets in immuno-oncology. It has four molecules in clinical trials (ES002, ES101, ES102, and ES104), and endeavors to clinically advance at least one innovative molecule a year. Founded and managed by a team of biopharma industry leaders, Elpiscience is backed by renowned investors including, Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, DYEE Capital and Cormorant Asset Management. Learn more at elpiscience.com.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces CDE Clearance of IND Application for Anti-CD39 Monoclonal Antibody ES002Job descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, today announced that the Center of Drug Evaluation (CDE) has cleared Elpiscience’s Investigational New Drug Application (IND) for ES002 to initiate a Phase I clinical trial in China.
ES002 is a proprietary anti-CD39 monoclonal antibody (mAb), which has demonstrated highly potent single-agent anti-tumor activity showing a significant reduction in tumor size in in vivo pharmacology studies.
“We are delighted that our IND application for ES002 is approved by the CDE, which follows the earlier IND and Phase I clinical study initiation in the US,” said Dr. Steve Chin, CMO of Elpiscience. “Robust preclinical data suggests that our anti-CD39 mAb has a potential best-in-class profile, including a strong effect on T cell function and superior enzymatic inhibition. We look forward to initiating the study in China and potential clinical benefits ES002 may offer for patients with solid tumors worldwide.”
About ES002:
ES002 is an anti-CD39 mAb designed to promote anti-tumor immunity. CD39 is a key enzyme regulating the production of adenosine, a critical immune suppressor. By blocking CD39 function, ES002 also stabilizes pro-inflammatory extracellular ATP (eATP) and restores anti-tumor immunity within the tumor microenvironment. ES002 demonstrated highly potent single-agent anti-tumor activity in in-vivo pharmacology studies and is currently in a Phase 1 clinical trial in the US (NCT05075564).[JC1]
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focused on innovating and developing next-generation immunotherapy. The company has a robust pipeline of globally innovative molecules, covering wide range of targets in immuno-oncology. It has four molecules in clinical trials, including ES104, ES101, ES102, and ES002. Founded and managed by a team of biopharma industry leaders and scientists, Elpiscience is backed by renowned investors including, Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, DYEE Capital and Cormorant Asset Management. Elpiscience endeavors to advance at least one innovative molecule into the clinic each year to benefit cancer patients worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces First Patient Dosed in US Phase I Clinical Trial of Anti-CD39 Monoclonal Antibody ES002 for Treatment of Advanced Solid TumorsJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, today announced that the first patient has been dosed in a U.S. multi-center, Phase I clinical trial, evaluating the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary clinical activity of ES002, the company’s proprietary anti-CD39 monoclonal antibody (mAb), that is being developed for the treatment of advanced solid tumors (NCT05075564). Elpiscience received U.S. Food and Drug Administration (FDA) IND clearance for ES002 in September 2021.
“We are very pleased to see ES002 enter clinical trial testing in the United States,” said Steve Chin, CMO of Elpiscience. “This is an important milestone for Elpiscience as we seek to develop innovative and differentiated cancer immunotherapies. We look forward to announcing additional clinical milestones throughout 2022 as Elpiscience expands its pipeline with a steadfast commitment to advance at least one innovative molecule into the clinic each year.”
ES002 has demonstrated highly potent single-agent anti-tumor activity showing significant reduction in tumor size and weight in in-vivo pharmacology studies.
About ES002:
ES002 is an anti-CD39 mAb designed to promote anti-tumor immunity. CD39 is a key enzyme regulating the production of adenosine, a critical immune suppressor. By blocking CD39 function, ES002 also stabilizes pro-inflammatory extracellular ATP (eATP) and restores anti-tumor immunity within the tumor microenvironment. ES002 demonstrated highly potent single-agent anti-tumor activity in in-vivo pharmacology studies.
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focused on innovating and developing next-generation immunotherapy. The company has a robust pipeline of globally innovative molecules, covering wide range of targets in immuno-oncology. It has four molecules in clinical trials, including ES104, ES101, ES102, and ES002. Founded and managed by a team of biopharma industry leaders and scientists, Elpiscience is backed by renowned investors including, Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, DYEE Capital and Cormorant Asset Management. Elpiscience endeavors to advance at least one innovative molecule into the clinic each year to benefit cancer patients worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present at the 40th Annual J.P. Morgan Healthcare ConferenceJob descriptionJob description
Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation cancer immunotherapies, today announced that its co-founder and chief executive officer, Dr. Darren Ji, will present at the J.P. Morgan 40th Annual Healthcare Conference. Details of the presentation are as follows:
Time: Tuesday, January 11, 2022, 8:00 -8:25am EST
Location: Private track 1
Type: Presentation
Speaker: Darren Ji
The J.P. Morgan Annual Healthcare Conference is the largest healthcare investment symposium in the industry and will take place virtually from January 10 to 13, 2022.
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focused on innovating and developing next-generation immunotherapy to benefit cancer patients worldwide. The company has a robust pipeline of globally innovative molecules, covering wide range of targets in immuno-oncology. It has four molecules in clinical trials (ES002, ES101, ES102, and ES104), and endeavors to clinically advance at least one innovative molecule a year. Founded and managed by a team of biopharma industry leaders, Elpiscience is backed by renowned investors including, Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, DYEE Capital and Cormorant Asset Management.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present Two Programs at The Society for Immunotherapy of Cancer (“SITC”) 2021 Annual MeetingJob descriptionJob description
Shanghai/Suzhou, China, and Maryland, US, October 22, 2021. Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company focusing on discovering and developing next-generation cancer immunotherapies, today announced that the company would present two exciting innovations at the 36thSociety for Immunotherapy of Cancer (SITC) Annual Meeting, which will be taken place both in person and virtually in Washington, D.C. from November 10 to 14, 2021.
Elpiscience will present:
1) a novel bispecific macrophage engager platform, and
2) an innovative anti-CD39/TGFβ-Trap bispecific antibody
The poster presentations are scheduled both online and offline.
Details of the Poster presentations are as follows:
Title:
A Novel Bispecific Macrophage Engager Antibody (BiME) Designed for Cancer Immunotherapy
Abstract No.:
# 272
Session Date:
7:00-20:00 EST, Nov. 12-14, 2021
First Author:
Dr. Dawei Sun, Elpiscience
Presenter:
Dr. Dawei Sun, Elpiscience
Title:
Creating an Immune-favorable Tumor Microenvironment by A Novel Anti-CD39/TGFβ-Trap Bispecific Antibody (ES014)
Abstract No.:
# 792
Session Date:
7:00-20:00 EST, Nov. 12-14, 2021
First Author:
Dr. Dawei Sun, Elpiscience
Presenter:
Dr. Hongtao Lu, Elpiscience
About ES014:
ES014, a dual acting molecule that binds CD39 and TGF-β simultaneously, was designed by fusing the TGF-β Receptor-II ectodomain to an antibody targeting CD39. The bi-specific molecule inhibits ATPase activity, resulting in the inhibition of the generation of immune-suppressive adenosine and promotion of immune-stimulating ATP while at the same time it also neutralizes immunosuppressive factor, TGF-β, aiming to create an immune-favorable tumor microenvironment. It has been demonstrated that ES014 can block Treg differentiation and significantly promote the survival and function of Teff cells. ES014 achieved a strong single agent anti-tumor efficacy in a PD-1 unresponsive mouse model.
About BiME:
BiME, is a proprietary bi-specific macrophage engaging platform developed by Elpiscience. By utilizing a SIRPα inhibitory antibody as a macrophage engager and activator, we have linked it to an anti-tumor-associated-antigen (TAA) antibody to directly activate macrophage phagocytosis towards the particular TAA cancer. ES028, a bispecific antibody targeting CLDN18.2 and SIRPα, is a unique biotherapeutic candidate coming out of the BiME platform. ES028 has demonstrated promising therapeutic efficacy in a pre-clinical animal model with a favorable tolerability profile. Different BIME bi-specific antibodies are being developed, utilizing different TAA antibodies to target different cancer types.
About Elpiscience:
Elpiscience is a global-clinical stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” tumors “hot”. The company has four assets in clinical stage (ES104, ES101, ES102 and ES002). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by renowned investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, Cormorant Asset Management and Superstring Capital. Elpiscience endeavors to advance at least one potentially innovative or differentiated global molecule into the clinic each year, providing clinical benefits to cancer patients worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces CDE Clearance of IND Application for ES104, Next-frontier Anti-angiogenesis DrugJob descriptionJob description
Shanghai/Suzhou, China, October 15, 2021. Elpiscience Biopharma, a global clinical-stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, today announced that Center of Drug Evaluation (CDE) has cleared Elpiscience’s IND application for ES104 to initiate a Phase I clinical trial in China.
ES104 is a bispecific antibody, targeting DLL4 and VEGF simultaneously. Based on the first in-human Phase 1 single agent dose escalation and expansion study in Korea, ES104 hasdemonstrated significant clinical activity as a stand-alone therapy. According to the data, among patients with advanced solid tumors with a median of three prior lines of systemic anticancer therapies (>60% of patients were previously treated with anti-VEGF antibodies containing regimens), the confirmed overall response rate (ORR) across all dose levels tested (0.3 – 17.5mg/kg) was 8%, and the clinical benefit rate (CBR) was 62%. The ORR at the Recommended Phase 2 Dose (RP2D) was 19%, and the CBR at the RP2D was 69%. Importantly, the response rate is almost three times that seen for current 3rd and 4th line therapies in patients with colorectal and gastric cancers. Meanwhile, ES104 was well tolerated across all doses evaluated, with no dose-limiting toxicities reported.
Dr. Steve Chin, M.D., CMO of Elpiscience, said “We are very pleased that CDE has cleared the China IND Application for ES104, overseas clinical data has demonstrated a preliminary testament to its potential to benefit heavily pr-treated patients with advanced solid tumors. We will move forward with this clinical program with an all-out effort, and we are eager to explore its therapeutic prospect in treatment for digestive tract cancer with a high incidence rate in China to fulfill a significant unmet medical need. Hopefully, the product will provide more feasible therapeutic intervention for benefit cancer patients”.
About ES104:
ES104 is a bispecific antibody that simultaneously blocks Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways, which are critical to angiogenesis and tumor vascularization. Pre-clinical and early clinical data of ES104 shows that blockade of both pathways provides robust anti-tumor activity across several solid tumors, including colorectal, gastric, cholangiocarcinoma, pancreatic and non-small cell lung cancer. Partial responses to ES104 as monotherapy have been observed in heavily pre-treated cancer patients, who were resistant to currently approved anti-VEGF therapies. ES104 has completed a Phase 1 monotherapy dose-escalation and expansion study. Phase 1b and Phase 2 combination studies are ongoing. Greater China Rights of ES104 were held by Elpiscience.
About Elpiscience:
Elpiscience is a clinical-stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” tumors “hot”. The company has four assets in the clinical stage (ES101, ES102, ES104 and ES002). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by renowned investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, Cormorant Asset Management and Superstring Capital. Elpiscience endeavors to advance at least one potentially innovative or differentiated global molecule into the clinic each year, providing clinical benefits to cancer patients worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces First Patient Dosed with ES102 in combination with Toripalimab in ChinaJob descriptionJob description
Shanghai/Suzhou, China, October 15, 2021. Elpiscience Biopharma, a global clinical-stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, today announced that the first patient has been dosed in a Phase 1 dose-escalation study in patients with advanced solid tumors in combination with TUOYI® (toripalimab). Data from single-agent dose-escalation and expansion shown that ES102 was well tolerated with a favorable safety profile and had a sign of preliminary anti-cancer activity.
Dr. Steve Chin, M.D., CMO of Elpiscience, said “We are looking forward to seeing ES102 in combo with anti-PD-1 to provide significantly improved clinical benefit to patients with advanced solid tumors”.
About ES102:
ES102 is a hexavalent OX40 agonistic antibody with a unique “first-in-class” design developed by Elpiscience. The special design of ES102 allows for efficient OX40 agonism even in the absence of exogenous cross-linking. ES102 induces much strong immune activation compared to the conventional bivalent OX40 antibodies. It has demonstrated impressive single-agent anti-tumor efficacy and synergy with anti-PD1/PD-L1 agents in preclinical models and has shown a favorable safety profile and preliminary anti-cancer activity.
About Elpiscience Biopharma:
Elpiscience is a clinical-stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” tumors “hot”. The company has four assets in the clinical stage (ES101, ES102, ES104 and ES002). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by renowned investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, Cormorant Asset Management and Superstring Capital. Elpiscience endeavors to advance at least one potentially innovative or differentiated global molecule into the clinic each year, providing clinical benefits to cancer patients worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES104 (CTX-009/ABL001) Clinical Data Presented at the 2021 AACR-NCI-EORTC International ConferenceJob descriptionJob description
1. ES104 was well tolerated and demonstrated single agent activity in heavily pre-treated patients with solid tumor who are resistant to anti-VEGF therapies, mostly of colorectal and gastric origins;
2. The structural differences between ES104 and other bispecifics targeting these pathways, as well as the affinities and avidities to their targets, may be the drivers for the differentiated safety and efficacy data observed;
3. The maximum tolerated dose (MTD) was not reached, and the recommended Phase 2 doses (RP2D) of ES104 were determined to be 10.0 and 12.5 mg/kg biweekly;
4. Overall response rate (ORR) of ES104 as a monotherapy across all doses tested (0.3 - 17.5 mg/kg) was 8% and the disease control rate (DCR) was 62% in patients treated at the 3rd and 4th line settings;
5. Treatment with ES104 at the RP2D (10.0 mg/kg and 12.5 mg/kg) led to 19% (n=3/16) ORR, not including an additional unconfirmed partial response, and a 69% DCR (n=11/16);
6. A Phase 1b study of ES104 in combination with chemotherapy and a Phase 2 study are underway.
BOSTON, Mass, USA, SEONGNAM, S. Korea, SHANGHAI/SUZHOU, China, Oct. 8th, 2021. Elpiscience Biopharma, Compass Therapeutics, Inc. (OTC:CMPX) and ABL Bio (KOSDAQ: 298380) presented today clinical trial data for ES104 (CTX-009/ABL001), a dual anti-angiogenic bispecific antibody targeting DLL4 and VEGF-A, at an oral plenary session during the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics (Abstract Number: 4749; Session Title: Plenary Session 2: New Drugs on the Horizon I).
The first in human Phase 1 single agent dose escalation and expansion study evaluated ES104 across nine dose levels. The study enrolled 45 heavily pre-treated patients with cancers primarily of colorectal and gastric origin. ES104 was well tolerated across all doses evaluated, with no dose-limiting toxicities reported. The most frequent treatment related adverse event was hypertension, observed in 17 patients of the 45 patients enrolled. Among those, 7 patients reported Grade 3 hypertension and the rest had Grade 1 or Grade 2. Only 4 mild cases of pulmonary hypertension were reported that were all reversible, and ES104 demonstrated significant clinical activity as a stand-alone therapy. The majority (87%) of the patients enrolled in the study had ECOG performance status of 1; 42% (n=19) were patients with gastric cancer and 40% (n=18) were patients with colorectal cancer with a median of three prior lines of systemic anticancer therapies. Importantly, 62% of the patients enrolled were previously treated with anti-VEGF antibodies containing regimens. There were 4 partial responses (including three partial responses confirmed by RECIST 1.1 and one partial response which was unconfirmed) and 20 stable diseases among 39 evaluable patients. The confirmed overall response rate (ORR) across all dose levels tested (0.3 – 17.5mg/kg) was 8%, not including 1 unconfirmed partial response, and the disease control rate (DCR) across all dose levels was 62%. The ORR at the recommended phase 2 doses (RP2D) of 10.0-12.5 mg/kg was 19% (n=3/16) not including one unconfirmed partial response, and the DCR at the RP2D was 69% (n=11/16).
“We are happy to see the next frontier of anti-angiogenic therapies, ES104 showing promising anti-tumor activity in a Phase 1 study as monotherapy,” said Steve Chin, MD, CMO of Elpiscience Biopharma, “We look forward to initiating the clinical trials in China to explore its therapeutic potential in the heavily pre-treated digestive tract cancer patients.”
“This is a significant clinical result because current approved anti-angiogenic drugs have little efficacy as a monotherapy. Furthermore, 94% of the colorectal patients and 58% of the gastric patients in this study were previously treated with Avastin® (bevacizumab) or Cyramza® (ramucirumab), respectively; the response rate is almost three times that seen for current 3rd and 4th line therapies in patients with colorectal and gastric cancers.” said Jeeyun Lee, MD, a Professor at the Samsung Medical Center, Seoul, South Korea and the principal investigator of the study.
“The responses to ES104 (CTX-009/ABL001) as a monotherapy in this refractory patient population combined with the excellent tolerability profile suggests that ES104 (CTX-009/ABL001) can become an important drug for a broad range of solid tumors” said Thomas Schuetz, MD, PhD, CEO and scientific Founder of Compass Therapeutics. “We look forward to developing ES104 (CTX-009/ABL001) in the United States and other geographies and to unlocking its full potential”. Compass Therapeutics of Boston, Massachusetts holds the global rights to ES104 (CTX-009/ABL001) with the exception of S. Korea (rights held by Handok) and China (rights were out-licensed to Elpiscience Biopharma).
“We are pleased to present the clinical data of ES104 (CTX-009/ABL001) for the first time at a prominent international conference,” said Sang Hoon Lee, PhD, CEO of ABL Bio. “ES104 (CTX-009/ABL001) has demonstrated its potential to benefit cancer patients, especially those that have been unable to experience improvements with standard treatments. We expect to further validate the therapeutic value of ES104 (CTX-009/ABL001) as it progresses through clinical trials in the U.S., China and South Korea.”
About ES104:
ES104 (CTX-009/ABL001) is a bispecific antibody that simultaneously blocks Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways, which are critical to angiogenesis and tumor vascularization. Pre-clinical and early clinical data of ES104 suggests that blockade of both pathways provides robust anti-tumor activity across several solid tumors, including colorectal, gastric, cholangiocarcinoma, pancreatic and non-small cell lung cancer. Partial responses to ES104 as a monotherapy have been observed in heavily pre-treated cancer patients, who were resistant to currently approved anti-VEGF therapies. ES104 has completed a Phase 1 monotherapy dose escalation and expansion study. Phase 1b and Phase 2 combination studies are ongoing.
About Elpiscience Biopharma:
Elpiscience is a clinical stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” tumors “hot”. The company has four assets in clinical stage (ES101, ES102, ES104 and ES002). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by renowned investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, Cormorant Asset Management and Superstring Capital. Elpiscience endeavors to advance at least one world-class molecule into the clinic each year, providing clinical benefits to cancer patients worldwide.
About Compass Therapeutics:
Compass Therapeutics, Inc. is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. Compass’ scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth. The company pipeline of novel product candidates is designed to target multiple critical biological pathways required for an effective anti-tumor response. These include modulation of the microvasculature via angiogenesis-targeted agents, induction of a potent immune response via activators on effector cells in the tumor microenvironment; and alleviation of immunosuppressive mechanisms used by tumors to evade immune surveillance. Compass plans to advance its product candidates through clinical development as both standalone therapies and in combination with proprietary pipeline antibodies based on supportive clinical and nonclinical data. The company was founded in 2014 and is headquartered in Boston, Massachusetts.
About ABL Bio:
ABL Bio, Inc. (KOSDAQ: 298380) is a clinical-stage biotechnology company developing antibody therapeutics for immune-oncology and neurodegenerative diseases. With internal R&D and global partnerships, ABL has developed multiple bispecific antibody platforms, such as ‘Grabody-T,’ ‘Grabody-I’ and ‘Grabody-B’ and built an innovative pipeline of multiple clinical and pre-clinical stage drug candidates. In the oncology area, ABL has developed Grabody-T, a modular 4-1BB engaging platform that has demonstrated superior efficacy and safety. In the neurodegenerative disorder space, ABL has developed Grabody-B, which is designed to maximize blood-brain barrier (BBB) penetration. Grabody-B is applicable to various CNS targets across a plethora of neurological disorders, potentially providing a breakthrough to address the high unmet medical needs in neurodegeneration.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces FDA Approval of IND Application for ES002 (anti-CD39 mAb)Job descriptionJob description
Shanghai/Suzhou/Maryland, September 10, 2021. Elpiscience Biopharmaceuticals, Inc. (“Elpiscience”), a global clinical-stage biopharmaceutical company focusing on discovering and developing next-generation cancer immunotherapies, today announced its investigational new drug (IND) application for ES002, an internally discovered novel antibody, has been approved by the U.S. Food and Drug Administration (FDA). A first-in-human clinical trial will be initiated in the U.S. to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary clinical activity of ES002 in patients with advanced solid tumors.
“Elpiscience is dedicated to revolutionizing cancer care by turning ‘cold’ tumors ‘hot’. ES002 is our first internally developed asset that is advancing into clinical trials in the U.S. and China. Dr. Hongtao Lu, co-founder and CSO of Elpiscience, said: “With a strong drug R&D engine and technology platforms, we aim to deliver sustainable innovative molecules to cancer patients worldwide. We look forward to seeing the distinctive features of ES002 to be translated into clinical benefits.”
“The IND clearance for ES002 is an important milestone for Elpiscience. This represents our commitment to advancing at least one world-class molecule into the clinic each year,” said Dr. Steve Chin, CMO of Elpiscience. “We hope ES002 will provide clinical benefits to cancer patients worldwide.”
About ES002:
ES002 is a highly promising, potential best-in-class anti-CD39 antibody designed to promote anti-tumor immunity. CD39 is a key enzyme regulating the production of adenosine, a critical immune suppressor. By blocking CD39 function, ES002 also stabilizes pro-inflammatory extracellular ATP (eATP) and restores anti-tumor immunity within the tumor microenvironment. ES002 demonstrated highly potent single-agent anti-tumor activity in in vivo pharmacology studies.
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” tumors “hot”. In addition to ES002, the company has three assets in clinical stage (ES101, ES102 and ES104). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by renowned investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments, Greater Bay Area Homeland Development Fund, CDH, Cormorant Asset Management and Superstring Capital. Elpiscience endeavors to advance at least one world-class molecule into the clinic each year, providing clinical benefits to cancer patients worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces CDE Clearance of IND Application for ES102 in Combination of ToripalimabJob descriptionJob description
SHANGHAI/SUZHOU, China, June 25, 2021. Elpiscience Biopharma Ltd., a clinical-stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, today announced that the Center of Drug Evaluation (CDE) has approved the IND application for ES102, a next generation anti-OX40 antibody, in combination with toripalimab (TUOYI®) an anti-PD1 antibody, in patients with advanced solid tumor.
In China, Elpiscience aimss to evaluate the safety, tolerability and explore early efficacy signal of ES102, the next-generation hexavalent anti-OX40 antibody, in combination with toripalimab for the treatment of advanced solid tumors. Now, ES102 mono dose escalation in advanced solid tumors is ongoing led by Professor Ying Cheng at Jilin Cancer Hospital.
In the US, single agent dose escalation for ES102 (INBRX-106) was completed. Besides, single agent dose expansion and dose escalation in combination with Keytruda are currently ongoing .Signs of durable anti-cancer activity were observed and ES102 as a single agent was well tolerated in phase I clinical trial.
Dr. Steve Chin, CMO of Elpiscience, said: “We are happy to explore the anti-tumor potentials of ES102 in combination with toripalimab together with Junshi Biosciences. We hope the combination could provide more treatment options to patients who cannot benefit from current therapies.”
About ES102
ES102 is a hexavalent OX40 agonistic antibody with a unique “first-in-class” design. The special design of ES102 allows for efficient OX40 agonism even in the absence of exogenous cross-linking. ES102 induces much strong immune activation compared to tetrameric or conventional bivalent OX40 antibodies, it has demonstrated impressive single agent anti-tumor efficacy and great synergy with anti-PD1/PD-L1 agents in preclinical models.
About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of 15 globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” into “hot” tumors. The company currently has three assets in clinical trials (ES101, ES102 and ES104), three in IND-enabling stage (ES002, ES014 and ES004). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by close to 20 top investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments and Great Bay Area Development Fund.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present at the Jefferies 2021 Healthcare ConferenceJob descriptionJob description
SHANGHAI/SUZHOU, China, June 2, 2021. Elpiscience Biopharma Ltd., a clinical stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, today announced that Dr. Darren Ji, Co-Founder and Chief Executive Officer of Elpiscience, will present an update on company status and R&D pipeline at the Jefferies 2021 Healthcare Conference at 8:30 p.m. Asia/Shanghai Time on Friday, June 4, 2021.
A live webcast of the Presentation can be retrieved through the link as below:
https://wsw.com/webcast/jeff174/elp/1695735.
About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of 15 globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” into “hot” tumors. The company currently has three assets in clinical trials (ES101, ES102 and ES104), three in IND-enabling stage (ES002, ES014 and ES004). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by close to 20 top investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments and Great Bay Area Development Fund.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Arlene Sharpe, MD, Ph.D.Job descriptionJob description
Dr. Arlene Sharpe, a renowned immunologist and Professor at Harvard Medical School. Dr. Sharpe is a former president of the American Association of Immunologists. In 2017, Dr. Sharpe received the Warren Alpert Foundation Prize with others for their collective contributions to the development of immune checkpoint blockade, a novel cancer therapy transforming the landscape of cancer treatment.
She is currently Chair of the Department of Immunology at Harvard Medical School and co-director of the Evergrande Center for Immunologic Diseases at Harvard Medical School and Brigham and Women’s Hospital.
Dr. Sharpe earned her M.D. and Ph.D. degrees from Harvard Medical School.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces the Completion of $105 Million Series C FinancingJob descriptionJob description
SHANGHAI/SUZHOU, China, May 13, 2021. Elpiscience Biopharma, a clinical stage biopharma focusing on the discovery and development of next-generation cancer immunotherapies, announced today the closing of Series C round of financing of 105 million USD. This round of financing was led by the Greater Bay Area Homeland Development Fund, with participation from Cormorant Asset Management, Maison Capital, Superstring Capital, Pluto Connection Limited, Unifortune Fund etc. Existing investors, including Lilly Asia Ventures, CDH Investments, Dyee Capital and Oriza Holdings continued to invest in this round of financing.
Proceeds raised from this round will be primarily used to expand the global reach of Elpiscience's R&D strategy, i.e. as a start, to advance Elpiscience’s innovative molecules into clinical studies in the US. Part of the proceeds will also be used for exploring novel mechanisms of cancer immunotherapy and fueling more strategic partnerships.
“We are thrilled to have the continued support and endorsement from the top tier investors. It’s been a remarkable 3.5 years,” said Dr. Darren Ji, Chairman and CEO of Elpiscience, “Elpiscience is determined to become a innovation leader in developing the next generation of cancer immunotherapies. We are committed to bringing at least one world-class molecule into the clinic each year to benefit cancer patients globally.”
About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on innovating and developing the next generation of cancer immunotherapy. Elpiscience has developed a pipeline of 15 globally innovative molecules, covering a wide range of targets with a particular focus on turning “cold” into “hot” tumors. The company currently has three assets in clinical trials (ES101, ES102 and ES104), three in IND-enabling stage (ES002, ES014 and ES004). Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by close to 20 top investors such as Lilly Asia Ventures, Hillhouse Capital, Hyfinity Investments and Great Bay Area Development Fund.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Jason John Luke, M.D.Job descriptionJob description
Dr. Luke is the Chief Medical Officer of Strand Therapeutics and an internationally recognized expert in cancer immunotherapy and drug development. Prior to joining Strand, Dr. Luke served as Associate Professor of Medicine at the University of Pittsburgh, where he was Director of the Immunotherapy and Drug Development Center and Associate Director for Clinical Research at the UPMC Hillman Cancer Center.
Earlier in his career, Dr. Luke held academic and clinical positions, including Assistant Professor of Medicine at the University of Chicago, Attending Physician at Brigham and Women’s Hospital in Boston, and Instructor in Medicine at Harvard Medical School.
Dr. Luke received his Doctor of Medicine from the Rosalind Franklin University of Medicine and Science/Chicago Medical School and obtained a Bachelor's degree in Microbiology from the University of Iowa. He completed his training as a resident at Boston University Medical Center. Dr. Luke undertook the fellowship of medicine at Weill Cornell Medical Center, New York, and the fellowship of Medical Oncology at Memorial Sloan-Kettering Cancer Center, New York.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience and TRIGR Announce an Exclusive License Agreement of TR009 in Greater ChinaJob descriptionJob description
1. Transaction to immediately accelerate clinical development of TR009 in Greater China and combine with Elpiscience’s deep pipeline of next generation immunotherapies;
2. TRIGR and Elpiscience intend to harmonize future clinical development globally across multiple DLL4 overexpressed solid tumors;
3. TRIGR to receive a $7 million upfront cash payment, $110 million in potential milestones, and royalties;
IRVINE, CA and SHANGHAI, China, January 20, 2021. TRIGR Therapeutics, Inc. (“TRIGR”), a US based clinical stage biopharmaceutical company focused on the development of multi-targeted angiogenic and immunomodulatory bispecific antibodies for oncology and certain ischemic indications and Elpiscience Biopharmaceuticals, a leading China based clinical stage company focused on developing next generation of cancer immunotherapies announced today that they have entered into an exclusive licensing agreement for the development and commercialization of TR009 in mainland China, Hong Kong, Macau and Taiwan.
Under the terms of the agreement, TRIGR will receive an upfront cash payment of $7 million and is eligible to receive additional development and commercial milestones of $110 million plus royalties on annual net sales of TR009. Elpiscience obtains the exclusive development and commercialization rights of TR009 for Greater China across all oncology indications and will lead the clinical development and commercialization by leveraging on its translational science, clinical and regulatory experience to accelerate the path to approval of TR009 in its territory. A Joint Development Committee (JDC) will be formed to collaborate and harmonize TR009’s clinical development globally.
George Uy, Founder and Chief Executive Officer of TRIGR stated, “TRIGR was formed with a vision to globalize drug development and accelerate transformative treatment to patients worldwide. Our partnership with Elpiscience in Greater China is a significant milestone in this endeavor. We would also like to acknowledge and thank ABL Bio (KOSDAQ: 298380), the originator of TR009 (aka ABL001/NOV1501) for their support in the Phase 1 clinical studies and immense contribution to this program, along with HANDOK (KOSDAQ:095700), National Oncoventure (NOV) and Binex (KOSDAQ:053030).”
Dr. Darren Ji, co-Founder, Chairman and Chief Executive Officer of Elpiscence stated, “Elpiscience is committed to delivering the next generation of cancer immunotherapy to patients who cannot benefit from current treatment. TR009 has demonstrated impressive single agent activity in tumor types that are poorly responsive to present immunotherapies and is highly synergistic with our next generation immune modulators. The addition of TR009 to our pipeline will further strengthen our efforts in tackling “cold tumors” and delivering efficient therapeutics.”
Miranda Toledano, Chief Operating/ Financial Officer of TRIGR stated “Emerging data from our Phase 1 studies demonstrate TR009 is a highly differentiated dual angiogenic blocker, safely inhibiting both DLL4-mediated Notch signaling and VEGF with activity across several DLL4 overexpressed tumors. We believe this profile could position TR009 as a next generation angiogenic flagship molecule with multiple rational combinations and sBLA opportunities.”
Dr. Steve Chin, Chief Medical Officer of Elpiscience added, “We were particularly encouraged by TR009 data in heavily pre-treated tumors refractory to anti-VEGF such as colorectal and gastric cancers. We look forward to working together with the TRIGR team to expedite delivery to patients.”
About TR009
TR009 (aka ABL001/NOV1501) is an anti-VEGFxDLL4 bispecific antibody, which is composed of an anti-VEGF antibody backbone C-terminally linked with a proprietary DLL4-targeting single-chain variable fragment. Data from TR009’s ongoing Phase 1 Dose Escalation/Expansion Monotherapy and Phase 1B Combination studies (n~60 patients) demonstrate a 67% Clinical Benefit Rate, with deep and sustained partial responses per RECIST criteria in heavily pre-treated colorectal and gastric cancer patients that have failed at least 3 lines of prior therapy and become resistant to multi-VEGF (Avastin®, Stivarga®, Cyramza®), EGFR, anti-PD-1/PD-L1 and chemotherapies. The Phase 1B results testing the safety of TR009 in combination with irinotecan or paclitaxel, have also shown deep and sustained partial responses in difficult to treat 3rd/ 4thline Intrahepatic Cholangiocarcinoma (Biliary Tract Cancer) and Non-Small Cell cancer patients that have failed multiple lines of chemo, biological therapy, and anti-PD-1. TR009 has been safety administered up to 17.5mg/kg dose with no dose limiting toxicities (DLT). In contrast to historical DLL4 and other Notch targeted therapies, the administration of TR009 has not been hampered by pulmonary hypertension or other cardiac toxicities. Further updates on these studies will be provided later in 2021.
About TRIGR Therapeutics
TRIGR is an emerging biotechnology company in the field of next generation cancer therapies that was incorporated in April 2018 and managed by biopharmaceuticals industry veterans. TRIGR focuses on clinical development and commercialization of targeted and immuno-modulatory drugs with validated mechanism of action and novel formats for the US, European and Asian markets. The Company’s pipeline includes a clinical stage dual-angiogenesis bispecific antibody program (TR009) and 2 first in classpre-clinical 4-1BB conditional agonist bispecific antibodies paired with BCMA (TRIA001) and B7H-IO (undisclosed checkpoint, TRIA002). TRIGR Therapeutics holds the global rights to TR009 (a.k.a ABL001/NOV1501), outside of the Republic of Korea. ABL Bio (KOSDAQ: 298380), a premier biotechnology company focused on cancer and neurodegenerative diseases, licensed the worldwide rights to TRIGR in November 2018.
TRIGR Contact: Miranda Toledano, Chief Operating/Financial Officer, Miranda.toledano@TrigrRx.com www.trigrrx.com
About Elpiscience Biopharmaceuticals
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Its clinical assets, ES101 and ES102 are completing Phase I clinical trials, while three in-house developed candidates with first-in-class and best-in-class potential are currently in IND-enabling studies. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies.
Elpiscience Contact: Judy Shi, Head of Business Development, judyshi@elpiscience.com http://www.elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Xiaoli WuJob descriptionJob description
Xiaoli Wu is head of quality assurance department of Elpiscience. She is a dedicated quality assurance professional whose experience spans from GLP to commercial cGMP over the past two decades. Prior to joining Elpiscience, Xiaoli was QA Director at Huaota BioPharma responsible for establishing quality management system.
Xiaoli holds a Bachelor's degree in Pharmaceutical Engineering from Hefei University of Technology in China.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present at 39th Annual J.P. MORGAN Healthcare ConferenceJob descriptionJob description
Shanghai, China, January 11, 2021. Elpiscience Biopharma Ltd., a clinical stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, today announced that Co-Founder and Chief Executive Officer Dr. Darren Ji will present an update on company status and R&D pipeline at 39th Annual J.P. Morgan 2020 Healthcare Conference, on Jan 13th 11:35am ET (Jan 14th 00:35am Beijing Time).
A live webcast of the Presentation will be retrieved through link as below:
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Its most advanced assets, ES101 and ES102 are both in Phase I clinical stage, and three in-house developed candidates with first-in-class potential are currently being evaluated in IND-enabling studies. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience was Named as One of the Cultivated Unicorn Enterprises of SuzhouJob descriptionJob description
Shanghai/Suzhou, China, 30th December 2020. Elpiscience Biopharma Ltd., a clinical stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, was named as one of the Cultivated Unicorn Enterprises of Suzhou, which testified that Elpiscienece’s leadership has gained recognition over the industry.
About Elpiscience:
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment.Its most advanced assets, ES101 and ES102 are both in Phase I clinical stage, and three in-house developed candidates with first-in-class potential are currently being evaluated in IND-enabling studies. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Vijay Kuchroo, DVM, Ph.D.Job descriptionJob description
Dr. Vijay Kuchroo, Samuel L. Wasserstrom Professor of Neurology at Harvard Medical School, Senior Scientist at Brigham and Women’s Hospital, and Co-Director of the Center for Infection and Immunity, Brigham Research Institutes, Boston. Dr. Kuchroo is also an institute member of the Broad Institute of MIT and Harvard and a senior investigator at its Klarman Cell Observatory (KCO) project and the Food Allergy Scientific Initiative (FASI) project. He is the founding director of the Evergrande Center for Immunologic Diseases at Harvard Medical School and Brigham and Women’s Hospital.
Dr. Kuchroo’s major research interests include autoimmune diseases– particularly the role of co-stimulation– the genetic basis of experimental autoimmune encephalomyelitis and multiple sclerosis, and cell surface molecules and regulatory factors that regulate induction of T cell tolerance and dysfunction. Notably, his laboratory first described TIM family of genes and identified Tim-3 as an inhibitory receptor expressed on T cells, which is now being exploited for cancer immunotherapy.
Dr. Kuchroo was also first to describe the development of highly pathogenic Th17 cells which have been shown to induce multiple autoimmune diseases in humans, and his paper on the development of Th17 is highly cited in immunology.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Philip D. Greenberg, MD.Job descriptionJob description
Dr. Philip D. Greenberg has been a major contributor to delineating the mechanism of T cell-mediated tumor cell killing. He is Professor of Medicine and Immunology at the University of Washington and head of the Program in Immunology at the Fred Hutchinson Cancer Research Center. Dr. Greenberg’s research focuses on elucidating and modulating T cell and tumor interactions, and developing cellular and molecular approaches to manipulate and genetically engineer T cell immunity. Dr. Greenberg was also co-founder of Juno Therapeutics (now part of Bristol-Myers Squibb), a U.S.-based biopharmaceutical company specialized in developing cell-based cancer immunotherapies.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces IND Submission of ES102Job descriptionJob description
Shanghai, China, 15th July 2020. Elpiscience Biopharma Ltd., a clinical stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies announced today that it has submitted an Investigational New Drug (IND) application to the Center of Drug Evaluation (CDE) in Beijing to support the initiation of a Phase I clinical trial of ES102 in China.
About ES102
ES102 is a hexavalent OX40 agonistic antibody with a unique “first-in-class” design. The special design of ES102 allows for efficient OX40 agonism even in the absence of exogenous cross-linking. ES102 induces much strong immune activation compared to tetrameric or conventional bivalent OX40 antibodies, it has demonstrated impressive single agent anti-tumor efficacy and great synergy with anti-PD1/PD-L1 agents in preclinical models. OX40, also known as CD134, is a member of the tumor necrosis factor (TNF) receptor family and is an important co-stimulator of T cell responses. Typically for TNF receptor family members, 3 molecules of OX40 bind to the trimeric OX40 ligand protein, activating downstream NF-κB, PI3K, and AKT pathways, which ultimately leads to increased cytokine production and is associated with enhanced T-cell expansion, differentiation, and the generation of long-lived memory T-cells.
About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules with its most advanced asset, ES101, already in clinical trials. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment. For more information please visit: http://www.elpiscience.com.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Thomas Gajewski, MD, Ph.D.Job descriptionJob description
Dr. Thomas Gajewski, a leading scholar in immunotherapy and AbbVie Foundation Professor of Cancer Immunotherapy at the Ben May Department for Cancer Research, and Professor of Pathology and Medicine at the University of Chicago Medical School. He is a former president of the Society for Immunotherapy of Cancer, and leads the Immunology and Cancer program of the University of Chicago Comprehensive Cancer Center.
Dr. Gajewski is known for his work on the development, activation, and regulation of T cells, and preclinical and translational studies on anti-tumor immunity and cancer immunotherapy, with a focus on melanoma. Dr. Gajewski also guides the development of immune-based therapies for other cancers, using new laboratory data on how the immune system is regulated to develop novel clinical trials.
Dr. Gajewski is the co-founder of Jounce Therapeutics and Pyxis Oncology, both U.S.-based biotechnology companies dedicated to the development of novel cancer immunotherapies.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Clinical collaboration with Junshi BiosciencesJob descriptionJob description
Shanghai, China, May 28th, 2020. Elpiscience Biopharma Ltd., a clinical stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies announced today that it entered a clinical collaboration with Junshi Biosicences (HKEX: 1877) to evaluate the safety, tolerability and explore early efficacy signal of its ES102, a next generation OX40 agonistic antibody, in combination with Junshi Biosciences’ toripalimab (TUOYI®), an anti-PD-1 mAb and immune checkpoint inhibitor for the treatment of solid tumors and hematological malignancies.
ES102 is a unique hexavalent OX40 agonistic antibody which has demonstrated strong single agent anti-tumor efficacy and great synergy with anti-PD1 agents in preclinical models. ES102 is currently being evaluated in dose escalation study in the US, while Elpiscience is planning clinical trials of ES102 in China, where combination with JS001 is part of the trial design.
Dr. Hongtao Lu, CSO of Elpiscience, said “We are happy to enter into a collaboration with Junshi Biosciences to evaluate ES102 and toripalimab combo in clinical trials. In China, TUOYI® is the first marketed anti-PD-1 mAb developed by a Chinese company, and Junshi Biosciences is also one of the front-runners in the IO field. We hope the promising preclinical results of ES102 and toripalimab combo could translate clinically and provide more treatment options to patients who cannot benefit from current therapies.”
Dr. Hui Feng, COO of Junshi Biosciences, said: “We have conducted extensive collaborations globally, to enhance clinical efficacy of toripalimab with innovative combination therapy. Compared with conventional OX40 antibodies, ES102 with unique design induces much strong immune activation and also demonstrates anti-tumor efficacy with PD-1 mAb in preclinical models. We expect the great potential of the ES102 and toripalimab combo therapy could be tested as soon as possible in clinical trials.”
About ES102
ES102 is a hexavalent OX40 agonistic antibody with a unique “first-in-class” design. The special design of ES102 allows for efficient OX40 agonism even in the absence of exogenous cross-linking. ES102 induces much strong immune activation compared to tetrameric or conventional bivalent OX40 antibodies, it has demonstrated impressive single agent anti-tumor efficacy and great synergy with anti-PD1/PD-L1 agents in preclinical models. OX40, also known as CD134, is a member of the tumor necrosis factor (TNF) receptor family and is an important co-stimulator of T cell responses. Typically for TNF receptor family members, 3 molecules of OX40 bind to the trimeric OX40 ligand protein, activating downstream NF-κB, PI3K, and AKT pathways, which ultimately leads to increased cytokine production and is associated with enhanced T-cell expansion, differentiation, and the generation of long-lived memory T-cells.
About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules with its most advanced asset, ES101, already in clinical trials. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment. For more information please visit: http://www.elpiscience.com.
About Toripalimab
Toripalimab is an anti-PD-1 monoclonal antibody developed by Junshi Biosciences. Toripalimab received its first approval for 2nd line treatment of metastatic melanoma on December 17, 2018 in China and was commercially launched in February 2019.
About Junshi Biosciences
Established in 2012, Junshi Biosciences is committed to developing first-in-class and best-in-class drugs through original innovation and becoming a pioneer in the area of translational medicine to provide patients with effective and affordable treatment options. On December 24, 2018, Junshi Biosciences was listed on the Main Board of the Stock Exchange of Hong Kong with the stock code: 1877.HK. The Company has established a diversified R&D pipeline comprising 21 drug candidates with therapeutic areas covering cancer, metabolic diseases, autoimmune diseases, neurologic diseases, and Infectious disease. Product types include monoclonal antibodies, fusion proteins, antibody-drug conjugates, and small molecule drugs. With a combined 33,000L fermentation capacity in two GMP-facilities at Shanghai and Suzhou, Junshi has established the manufacturing infrastructure to support commercialization and provide our partners and patients with high-quality products through a global supply chain network. For more information, please visit: www.junshipharma.com.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Competitive Reward PackageJob descriptionJob description
We offer market competitive compensation and benefit package, as well as long term incentives to attract and retain great talents.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
BiME®
B Cell DepletionJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Development OpportunitiesJob descriptionJob description
We provide abundant professional development opportunities and platforms, including general skills development training, talent program, cross team learning and sharing, and Leadership Academy etc.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present at the Jefferies 2020 Healthcare ConferenceJob descriptionJob description
Shanghai, China, May 26th, 2020. Elpiscience Biopharma Ltd., a clinical stage biotech company focusing on the discovery and development of next-generation cancer immunotherapies, today announced that Dr. Darren Ji, Chief Executive Officer, will present an update on company business and R&D pipeline at the Jefferies 2020 Healthcare Conference, at 21:30 Beijing China time (9:30am ET, U.S.), on June 4th.
A live webcast of the Presentation can be retrieved through the link as below: http://wsw.com/webcast/jeff126/elp/
About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules with its most advanced assets, ES101 and ES102, already in clinical trials. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment. For more information please visit: http://www.elpiscience.com.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Protein Chemistry Scientist Discovery Shanghai ChinaJob descriptionJob description
- Antibody developability assessment is mandatory, including antibody’s structural characterization, including intact mass, sequence confirmation, PTM and Glycan profiling, stability characterization, including: SDS-PAGE, SEC, HIC, CE-SDS and iCIEF.
- Recombinant protein purification on AKTA or other high-throughput antibody purification system is required occasionally.
- Keep laboratory records and present data to the project team.
Job requirements- Ph.D. or M.Sc. in pharmaceutical analysis, protein chemistry, biology, biochemistry, structural biology or related fields.
- Protein QC experience including SEC/HIC/Glycan profiling/CE-SDS/iCIEF by using HPLC/UPLC/Maurice. Familiar with different chromatography methods to analyze protein is preferred.
- Experience in protein conformational and thermal stability analysis is a plus, Tm /Tagg/Tonset /KD detection.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES304Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
ElpiStar RecognitionJob descriptionJob description
Elpistar is a unique program to encourage cross team collaboration and behaviors that reflect our core values, and recognize them in a timely manner.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Appoints Dr. Steve Chin as Chief Medical OfficerJob descriptionJob description
Shanghai, January 6, 2020. Elpiscience Biopharma, Ltd., a clinical-stage biopharmaceutical company announces the appointment of Steve Chin, M.D. as Chief Medical Officer.
Dr. Chin brings to the company many years of experience in medical oncology and clinical development in the pharmaceutical industry.

Prior to joining Elpiscience, Dr. Chin was a Senior Medical Director of immuno-oncology, Global Clinical Development at AstraZeneca. There, Dr. Chin provided leadership and expertise to enable new investment decisions in oncology therapeutic development, which involved the successful development of clinical strategy, determining the direction of regulatory interaction and coordination/execution of new clinical trials. Prior to his career at AstraZeneca, Dr. Chin was a Global GI Oncology Lead at Eli Lilly. He was involved in the transfer of cetuximab (Erbitux) full rights in North America from BMS to Lilly, led regulatory submission for Erbitux label updates, and fulfilled Erbitux post-marketing commitment.
Dr. Chin started his career as a medical oncologist and served as an assistant professor in the Division of Hematology/Oncology at the Medical University of South Carolina in Charleston. He was a clinical investigator with a special interest in cancer therapeutic development and correlative studies. Over his career, Dr. Chin has served as PI or Co-I in >20 active clinical trials.
Dr. Darren Ji, CEO and Co-Founder of Elpiscience stated, “We are elated to have Steve join us as Chief Medical Officer on our journey to find truly effective therapies in cancer immunotherapy. Steve brings abundant experience in both medical practices and drug development in the oncology space. His joining is extremely timely as we are rapidly advancing our clinical programs both in China and across the world. Steve’s extensive global clinical development experience in cancer immunotherapy will significantly strengthen our clinical development capability and accelerate the development of next-generation immunotherapy for patients.”
Dr. Steve Chin commented, “I’m thrilled about the rapid progress that Elpiscience has made in establishing a broad portfolio and an expanding pipeline of immuno-oncology molecules. It is my great honor to be a part of Elpiscience. I look forward to working with this exceptional team to drive the development of next-generation immuno-oncology therapeutics for the benefit of cancer patients in China and worldwide.”
About Elpiscience
Elpiscience is a clinical-stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience has a pipeline of more than 10 innovative molecules with its most advanced asset, ES101, already in clinical trials. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES302Job descriptionJob description
ES302: Next-Generation Bispecific Antibody for IBD Therapy
Our innovative TL1A and IL23p19 bispecific antibody represents a dual-targeting approach in the treatment of inflammatory bowel disease (IBD). Currently in the preclinical stage, this molecule is designed to simultaneously block two critical cytokine pathways, offering high potency and enhanced therapeutic efficacy. Engineered for subcutaneous administration, it boasts a long-acting profile and superior resistance to enzymatic degradation, ensuring sustained therapeutic action. By targeting both TL1A and IL23p19, our bispecific antibody aims to provide a more comprehensive and effective treatment option for patients with IBD, addressing the complex pathophysiology of the disease.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Diverse Sport ClubsJob descriptionJob description
At Elpiscience we believe physical fitness and mental health go hand in hand. Diverse sport clubs are established to help colleagues to enjoy an energetic and fun life.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Completes Series B Financing of USD $100 MillionJob descriptionJob description
December 28, 2019, Shanghai, China, Elpiscience Biopharma announces the completion of Series B financing of 100 million USD. The round was led by Hyfinity Investments, with participation from Tencent, GTJA Investment Group, Dyee Capital, Oriza Holdings, Ming Bioventures, WisdoMont, Parkway Global and others. Existing investors, including Lilly Asia Ventures, Hillhouse Capital Group, and CDH Investments continued to invest in this round of financing. The proceeds from Series B will primarily be used to advance highly innovative immunotherapy candidates such as ES101, ES102 and ES002 through pre-clinical and clinical development. The Company will also use these funds to expand its innovative product pipeline through internal discovery and global partnering.
Elpiscience is a biopharmaceutical research and development company that strives to lead the next revolution of cancer immunotherapies. Built on its deep understanding of tumor biology and immunology, and with its highly efficient execution capabilities, Elpiscience has developed a globally competitive pipeline of 12 products in just two years, which is highly recognized not only by institutional investors but also by the commercial banks. Two weeks ago in Suzhou, Elpiscience announced the signing of a 150 million RMB collaboration with two major banks, Bank of China and Agricultural Bank of China. The fund will be used to establish Elpiscience's GMP manufacturing capabilities. This signifies a major milestone of Elpiscience towards late stage and commercial development of antibody therapeutics.
Dr. Darren Ji, Elpiscience’s co-founder and CEO, commented, “We are pleased to be recognized by top investors during this round of financing. Their trust is truly appreciated, especially in this challenging year of private investment. We are particularly grateful towards Hyfinity Capital for their leading role and warmly welcome many other seasoned investors who participated in this Series B round. The participation of these top-notch investors greatly empowers us to continuously pursue the exciting journey of developing innovative drugs. We will stay committed to carefully exploring new scientific discovery towards finding effective therapies for cancer patients.”
Dr. Sylvia Xin He, Hyfinity Investments’ Managing Partner, commented, “Elpiscience has a deep understanding of immunotherapy and focuses on the truly global innovative targets. The team at Elpiscience excels in execution, international collaboration, and innovative drug discovery and research in the field of tumor immunology. The rapid progress from preclinical to clinical development, the establishment of an integrated R&D team and the system, and their global network and collaboration with leading academic institutions and industry partners testify to the team’s strength. Immunotherapy is one of the vertical foci for Hyfinity Investments, a field that we have already deeply cultivated for the last two years. We are honored to join and support Elpiscience in its pursuit of innovative cancer immunotherapy and Hyfinity is committed to assisting Elpiscience in its great endeavors.”
About Elpiscience:
Elpiscience is a clinical-stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpscience has a pipeline of more than 10 innovative molecules with its most advanced asset, ES101, already in clinical trials. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment. China Renaissance is financial advisor for Elpiscience.
About Hyfinity Investments:
Hyfinity Investments is led by senior partners from top-tier investment institutions in China, with years of experience in healthcare investment, local operation, and overseas licensing. Hyfinity Investments is devoted to advancing global innovations and leveraging advantages in China, including the high unmet needs and rich clinical resources in China. Hyfinity aims to foster industry leaders through the convergence of global resources.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Great Working EnvironmentJob descriptionJob description
We believe that happy employees are productive employees. Elpisicence is recognized and loved for its open, transparent, and inspiring environment for science and creativity.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
BiME® FranchiseJob descriptionJob description
BiME® Franchise
Building on the BiME® platform, we are advancing several programs to fully leverage its innovative potential. Our BiME pipeline targets tumors with a high abundance of tumor-associated macrophages (TAMs), including HCC, GC, and CRC, where effective therapies remain limited.The novel bispecific antibody macrophage engagers generated from this platform have the potential to transform clinical practice by reactivating macrophages and enhancing anti-tumor immunity in challenging cancer types.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Present ES002/ES004 Data at SITC 2019Job descriptionJob description
Shanghai September 19th 2019, Elpiscience Biopharma, Ltd., a clinical stage immuno-oncology company, announced that it will present data of anti-CD39 and anti-SIRPα antibodies, ES002 and ES004, at the upcoming SITC conference which will be held from Nov. 7-10, 2019 in Washington DC.
ES002 is a potentially best-in-class anti-CD39 therapeutic antibody, which reverses the suppressive tumor microenvironment and demonstrates strong anti-tumor efficacy in preclinical models. Mechanistic study revealing a dependency on innate immune cells in ES002-mediated anti-tumor response will be presented.
ES004 presentation will provide preclinical data on 2 unique anti-SIRPα antibodies, ES004-B4 and ES004-N4, which are CD47-SIRPα interaction “blocker” and “non-blocker”, respectively. Both antibodies greatly enhance macrophage-mediated tumor cell destruction, yet through different mechanisms of actions.
Details of the poster presentations are as follows:
Poster # P271
Title: Anti-SIRPalpha antibodies exert anti-tumor activity in both CD47-dependent and CD47-independent manners
Date: Friday, Nov. 8, 2019, 7:00 am - 8:00 pm
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Received Second Prize in Merck’s Advance Biotech Grant ProgramJob descriptionJob description
Shanghai Sep 10th 2019, Elpiscience Biopharma, Ltd., announced that it received the Second Prize, which is valued at 1 million RMB, in the Advance Biotech Grant program sponsored by Merck. The program aims to help innovative biotech companies that are committed to addressing the challenges in bringing drugs from discovery to the market. Merck has started the EB Grant programs since 2014. 2019 marks the 7th time of this award.
Mr. Peter Wang, VP, Head of CMC at Elpiscience said: “We’re very honored to receive the Second Prize as a recognition of our innovation drive. Merck has always been an industry leader in providing quality products and services in the biopharmaceutical industry. At Elpiscience, we strive to bring transformative medicines to patients. Facing challenges, we’re committed to developing next generation cancer therapies through innovative approaches.”
 If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Translational Research Medicine Senior Scientist Discovery Shanghai ChinaJob descriptionJob description
- Design, plan and execute in vitro and ex vivo assays to provide translational data that bridges discovery thinking with clinical needs for our programs at various maturity states from target idea to screening and asset optimization prior to candidate selection.
- Identification of novel or most suitable biomarkers for patient selection or stratification as well as monitoring of pharmacodynamics in animal models and patients as needed for clinical studies.
- A team player mentality and interest in managing external CRO activities are required to connect with all internal partners and external collaborators to foster a productive culture and deliver results.
Job requirements- Master or PhD degree in biochemistry, cell biology, pharmacology or clinical sciences; candidates with immunology and/or oncology background are strongly preferred.
- Significant hands-on experience in assay development or biomarker development, 3D cultures systems or exosome biology.
- Extraordinary flexibility and ability to think independently but work collaboratively within a big multidisciplinary matrix team of a fast-growing biotechnology organization.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES019 (BiME®)Job descriptionJob description
ES019: Anti-SIRPα/PD-L1 BiME® Molecule for Solid Tumor Immunotherapy
Tumor-associated macrophages are major component of immune cells in the tumor micro-environment (TME) that express an array of effector molecules leading to the inhibition of anti-tumor immune responses. SIRPα is a myeloid-lineage inhibitory receptor primarily expressed on phagocytic macrophages and DCs, which inhibits phagocytosis through engagement of its ligand CD47 expressed on tumors and normal tissues.Leveraging our proprietary Bispecific Macrophage Engager (BiME®) technology, Elpiscience has developed ES019, a PD-L1/SIRPα bispecific antibody designed to simultaneously reactivate macrophages and T cells for tumor elimination. By targeting both tumor cells and immune cells, ES019 significantly enhances anti-tumor efficacy compared to monotherapy or combination treatments.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Advancement of Two Discovery Molecules into CMCJob descriptionJob description
Shanghai Aug 27th 2019, Elpiscience Biopharma, Ltd., a clinical stage immuno-oncology company, announces that two key molecules in innate immunity have entered into CMC development stage.
The two therapeutic candidates, anti-CD39 (ES002) and anti-SIRPa (ES004) antibodies, are potentially best-in-class molecules. They harbor the potential to address PD-1/PD-L1 non-responsive tumors by unleashing the power of innate immunity. Scientific data of the two molecules will be presented at the upcoming SITC conference in November 2019, Washington DC.
About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpscience’s first therapeutic product ES101 has entered into clinical trial, and the second innovative product ES102 is IND ready. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by top investors such as Lilly Asia Ventures, Hillhouse Capital and CDH Investment.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Project Scientist Discovery Shanghai ChinaJob descriptionJob description
- Provide technically expertise to design, develop and perform hits finding for drug discovery.
- Provide scientific input into experimental design and data interpretation.
- Work in a close matrix team with other capabilities/disciplines to perform MoA studies.
Job requirements- Ph.D./Master’s degree in biochemistry, cell biology, pharmacology or other related field, immunology background preferred.
- Familiar with immunological assays such as M1/M2, T cell proliferation, ADCC, ADCP, and so on, is a plus.
- Ability to work independently as well as collaboratively within a multidisciplinary team.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Multiple-Targeting StrategyJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Elpiscience: Official Opening of Shanghai R&D CenterJob descriptionJob description
On July 16th, 2019, Elpiscience Biopharmaceutical Ltd. (Elpiscience) announced the official opening of its Shanghai R&D Center. In less than two years since its inception, the company has established independent research and development and production platforms ranging from “target discovery”, “functional verification”, “multi-specific antibody construction” to “process optimization”. Elpiscience possesses a competitive pipeline, while also striving to collaborate and innovate with other world-class companies.
Elpiscience was privileged to host distinguished attendees including Chen Weiwei, deputy general manager of Zhangjiang (Group) Co., Ltd., Lou Qi, general manager of Zhangjiang Biomedical Base Development Co., Ltd., and representatives of biomedical enterprises, research institutes and investment institutions. The ceremony proceeded with inspiration speeches, laboratory tours, and gathering of industry experts.
‘Focus on science and create hope’. Elpiscience believes that the completion of Shanghai R&D Centre is an important milestone for its development. Elpiscience strives to advance the next-generation immunotherapeutics in order to benefit patients.

 If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
In Vivo Pharmacology Senior Scientist Discovery Shanghai ChinaJob descriptionJob description
- Lead by line function leader, to deliver an in vivo pharmacology strategy to support program needs; to deliver high quality in vivo pharmacology studies, data packages and study reports for assigned projects; to build in-house technical capabilities in a fast-paced work environment as necessary.
- To build strong collaborations with and external partners and CRO companies, and to be accountable for guiding CROs to deliver high quality studies.
Job requirements- Ph.D./Master/Bachelor’s degree in pharmacology or related fields; immunology and oncology background preferred but not mandatory.
- At least two years of proven in vivo pharmacology experience including CDX, PDX, GEMM, and humanized PBMC/CD34+ cell models. Solid training in cell culture, mouse handling, tumor inoculation, iv, ip, sc dosing, tumor measurements, humane sacrifice of animals and dissection of tumor and other tissues are mandatory. Additional expertise, e.g., ELISA, flow cytometry, histopathology, ‘omics approaches etc., are a plus.
- Highly motivated, independent thinking and highly collaborative with multidisciplinary matrix teams.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Cytokine Suppression & Innate Immune ModulationJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
ES101: First Patient Dosing in Phase I Clinical TrialsJob descriptionJob description
On June 28, 2019, Elpiscience Biopharmaceutical Ltd. announced first-patient dosing of first-in-class bispecific antibody ES101, targeting both PD-L1 and 4-1BB, at Shanghai East Hospital. This is the first time that the bispecific antibody drug has been administered to Chinese patients, marking the opening of a new era of tumor immunotherapy in China.
The recent progression of tumor immunotherapy has significantly benefited cancer patients. The advent of first-generation immunological checkpoint antibodies, represented by anti-PD-1/PD-L1, has brought unprecedented hope to patients, although there are still many unsolved complications. For instance, existing first-generation immune-therapies have low overall response rates. Therefore, there is a huge demand for more effective next-generation immunotherapies. Elpiscience is committed to innovate, and will promote ES101 for the first time in China.
ES101 has the potential to become the next-generation immunotherapeutic antibody. It will achieve this by executing the concept of “de-brake and add gas” for immune cell activation. De-braking is accomplished by blocking PD-1/PD-L1 checkpoint inhibitor interaction, whilst adding gas is achieved by 4-1BB-mediated immune cell engagement and activation.
Summary of ES101
ES101 is a tetravalent bispecific antibody that contains four binding domains, two targeting PD-L1 and two targeting 4-1BB, with a molecular weight being only two-thirds of that of a conventional antibody. Based on high PD-L1 expression in the tumor microenvironment and ES101’s unique antibody engineering, ES101 can conditionally activate 4-1BB upon binding to PD-L1. This would allow for more efficient and targeted tumor killing by immune cells.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Immuno-Oncology ApproachJob descriptionJob description
Elpiscience is dedicated to transforming cancer treatment by developing innovative immunotherapies that turn “cold” tumors “hot”. By unlocking differentiated biology, we aim to enhance crosstalk between innate and adaptive immunity, harnessing the full potential of the immune system to fight cancer .
Cancer Immunotherapy
Harnessing the immune system to fight cancer has been a scientific pursuit for over a century. In recent years, immune checkpoint inhibitors have revolutionized cancer treatment, offering patients powerful new options.
However, two key challenges remain: identifying novel biological pathways with clinical relevance and developing preclinical models that reliably predict therapeutic success.
At Elpiscience, we tackle these challenges by uncovering unique biological insights that can effectively activate the immune system. Our experienced research and development teams, supported by world-renowned scientists, are committed to advancing next-generation immunotherapies.
Our Systemic Dual Approach
To overcome the immune system’s failure to fight cancer, Elpiscience is committed to revolutionizing cancer treatment by adopting a holistic and systematic approach to immunotherapy. Since multiple mechanisms contribute to immune evasion, we focus on two key strategies to restore immune function and drive robust anti-tumor responses.
Removing Immune Suppressive Factors in the Tumor Microenvironment (TME)
Tumors create an immunosuppressive microenvironment that actively inhibits immune responses. Various immune and non-immune cells within the TME secrete factors that trigger inhibitory signals, forming a significant barrier to effective immunotherapy. Elpiscience develops targeted therapies to neutralize these suppressive mechanisms, enhancing the immune system’s ability to fight cancer.
Reinvigorating Exhausted T Cells
In cancer patients, prolonged antigen exposure leads to T cell exhaustion, reducing their ability to attack tumors. Restoring T cell function is essential for a sustained and potent immune response. Elpiscience designs therapies that modulate costimulatory and inhibitory signaling pathways, revitalizing T cells to generate stronger and more durable anti-tumor effects.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
In-LicensingJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Elpiscience and Bio-Techne Announce Strategic CollaborationJob descriptionJob description
Shanghai and Minneapolis, April 22, 2019, Elpiscience BioPharma and Bio-Techne Corporation today announced that the companies have entered into a strategic collaboration for the development of anti-cancer therapeutics. Under the terms of the agreement, Elpiscience will have access to multiple antibodies from Bio-Techne’s antibody library for use in preclinical, clinical and commercial pharmaceutical development.
As a biotech company committed to leading the innovation and development of the next generation of cancer immunotherapy, Elpiscience, in collaboration with Bio-Techne, a global leader in life sciences and molecular diagnostics, will expand mutual capabilities in the field of cancer immunotherapy. This strategic collaboration also aims to accelerate the development of new biologics to address unmet medical needs in Oncology.
Dr. Darren Ji, CEO of Elpiscience, said: “We are very happy to enter into a strategic collaboration with Bio-Techne in therapeutic antibody research and development. This collaboration will leverage the strengths of both companies in the field of anti-cancer therapeutics and speed up the development of new cancer immunotherapies in order to bring more efficient treatment to cancer patients worldwide.”
David Eansor, President of Bio-Techne’s Protein Sciences Segment commented “We are extremely excited to partner with Elpiscience in the development of novel cancer immunotherapies. It is our goal to increase our partnerships with therapeutics developers to unleash the potential of our vast library of high-quality antibodies towards the development of next generation immunotherapies.”
About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpscience’s first therapeutic product ES101 has entered into clinical trial, and the second innovative product ES102 is IND ready. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by top investors such as Lilly Asia Ventures, Hillhouse Capital and CDH Investment.
About Bio-Techne Corporation
Bio-Techne Corporation (NASDAQ: TECH) is a leading developer and manufacturer of high quality purified proteins and reagent solutions - notably cytokines and growth factors, antibodies, immunoassays, biologically active small molecule compounds, tissue culture reagents and T-Cell activation technologies. Bio-Techne's portfolio also includes protein analysis solutions, sold under the ProteinSimple brand name, offering researchers efficient and streamlined options for automated western blot and multiplexed ELISA workflow. These reagent and protein analysis solutions are sold to biomedical researchers as well as clinical research laboratories and constitute the Protein Sciences Segment. Bio-Techne also develops and manufactures diagnostic products including FDA-regulated controls, calibrators, blood gas and clinical chemistry controls and other reagents for OEM and clinical customers. Bio-Techne's genomic tools include advanced tissue-based in situ hybridization assays (ISH) for research and clinical use, sold under the ACD brand as well as a portfolio of clinical molecular diagnostic oncology assays, including the ExoDx® Prostate(Intellescore) test (EPI) for prostate cancer diagnosis. These diagnostic and genomic products comprise Bio-Techne's Diagnostics and Genomics Segment. Bio-Techne products are integral components of scientific investigations into biological processes and molecular diagnostics, revealing the nature, diagnosis, etiology and progression of specific diseases. They aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses. With thousands of products in its portfolio, Bio-Techne generated approximately $643 million in net sales in fiscal 2018 and has over 2,100 employees worldwide.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES009Job descriptionJob description
ES009: Potential Best-in-Class Monoclonal Antibody Targeting LILRB2
ES009 is a potential best-in-class monoclonal antibody targeting LILRB2 designed to reprogram inhibitory myeloid cells to create an immune favorable tumor microenvironment (TME).LILRB2 is a critical inhibitory receptor expressed on myeloid cells (including monocytes, dendritic cells, macrophages and neutrophils), mediated by functional ligands such as HLA-G which are mainly expressed on tumor cells. LILRB2-mediated immune suppression is regarded as one mechanism of anti-PD-(L)1 resistance.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Bispecific Antibody Assay Scientist Discovery Shanghai ChinaJob descriptionJob description
- Design, coordinate and conduct experimental procedures. Modify and adapt experimental protocols, techniques and procedures to specific project requirements.
- Perform in vitro functional assays for novel combinations and bispecific antibody proof of concept and mechanism of action (MOA) study.
- Study area will focus on immune-oncology field, especially in T cell biology, innate immunity (macrophage, NK cells), tumor microenvironment and cold tumor.
Job requirements- Ph.D. or master degree with 1+ year of working experience. Background in biochemistry, cell biology, oncology, immunology or related field.
- Experience/skills in cell based functional assays, especially in bispecific antibody related field such as CD3 based T cell killing assay, NK cell assay or macrophage-based phagocytosis and polarization assay.
- Excellent technical skill sets, especially for cell-based assay (cell culture, transient/stable transfection/transduction, reporter assay), and immunology assay (ELISA, FACS, MLR assay).
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
礼来亚洲基金Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
高瓴创投Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
汇鼎Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
大湾区共同家园发展基金会Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Cormorant Asset ManagementJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Our Partnering StrategyJob descriptionJob description
At Elpiscience, we aim to forge long-term, mutually beneficial strategic relationships with leading biopharmaceutical companies and academic institutions to accelerate the development of potentially transformative immunotherapies. Our entrepreneurial culture and unique operational advantages enable nimble decision-making and highly collaborative partnerships to efficiently drive global immunotherapy innovation.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience to Organize “2019 Shanghai Symposium – Novel Targets for Cancer Immunotherapy”Job descriptionJob description
Shanghai, March 14, 2019, Elpiscience Biopharma, Ltd. announces its first Symposium on “Novel Targets for Cancer Immunotherapy”, which will be held on March 24th, at Shanghai Kempinski Hotel.
The field of Cancer Immunotherapy is advancing in a breathtaking speed. The first generation of immune check point antibodies such as PD1/PD-L1, CTLA4 antibodies have revolutionized clinical practice in many cancer types. However a majority of cancer patients can still not benefit from the first generation of IO drugs. Novel targets and innovative approaches are in urgent demand to meet the unmet medical needs. The symposium brings together world leaders in immuno-oncology, to explore new therapeutic targets and approaches, to inspire and open up game-changing paths for the next generation of cancer immunotherapies.

About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpscience’s first therapeutic product ES101 has entered into clinical trial, and the second innovative product ES102 is IND ready. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by top investors such as Lilly Asia Ventures, Hillhouse Capital and CDH Investment.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES014Job descriptionJob description
ES014: Clinical-Stage First-in-Class Anti-CD39xTGF-β BsAb
ES014 is a first-in-class anti-CD39xTGF-β bispecific antibody (bsAb) that simultaneously targets the adenosine and TGF-β pathways, two major immunosuppressive mechanisms in the tumor microenvironment (TME).By simultaneously targeting CD39 and TGF-β, ES014 can inhibit the production of immunosuppressive adenosine, promote the formation of immunostimulatory ATP, and neutralize immunosuppressive cytokine TGF-β.It works by selectively delivering TGFβ “trap” to CD39-expressing immune cells, blocking CD39-mediated adenosine production, and removing TGFβ-driven immune suppression to restore anti-tumor immunity.Mechanism of Action If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Toxicologist Discovery Shanghai ChinaJob descriptionJob description
- Responsible for designing, coordinating and overseeing toxicology studies of company’s product portfolios.
- Coordinate with CROs and provide guidance and leadership in toxicology studies, and ensure the toxicology studies meet the guidelines of regulatory agencies of NMPA, FDA, EMEA and other agencies.
- Responsible for the toxicology sections of IND, BLA/NDA and other related documents for regulatory submissions.
Job requirements- Ph.D. in Toxicology, Pharmacology, Biology or related disciplines.
3+ years' toxicology study related experiences at a CRO or biotech companies. - 3+ years of experience as a study director/or equivalent in toxicology studies
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
AstellasJob descriptionJob description
On December 28 2023, Elpiscience Biopharma, Ltd. and Astellas Pharma Inc. (TSE: 4503) announced a research collaboration and license agreement for novel bi-specific macrophage engagers (BiME®;), ES019 and another program. The two companies will collaboratively conduct early-stage research for these two programs. Elpiscience will also grant Astellas the right to add up to two additional programs to be included in the collaboration. If Astellas exercises its option, Elpiscience will grant Astellas the exclusive right to further research, develop, manufacture and commercialize the products for each program.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
CompassJob descriptionJob description
In January 2021 we entered into an exclusive licensing agreement with TRIGR Therapeutics, now part of Compass Therapeutics, for the development, manufacture and commercialization of ES104/TR009, an investigational bispecific antibody targeting VEGF and DLL4, in China.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Clinical Trial Approval of ES101 in ChinaJob descriptionJob description
Shanghai, February 22, 2019, Elpiscience Biopharma, Ltd. announced today that the bispecific antibody ES101 has been approved by the Center for Drug Evaluation (CDE) for clinical trials in China. ES101 is a first-in-class, bispecific antibody targeting both PD-L1 and 4-1BB, where 4-1BB-induced T cell activation is dependent on the binding to PD-L1 in the tumor microenvironment. The combination of "de-brake" and "add-gas" ideas not only greatly improved the efficacy of the drug, but also avoided the toxicity that 4-1BB single agent may have. This would solve the problem that 4-1BB monoclonal antibodies have encountered in clinical development. In the mean time, on February 19th, Inhibrx, Elpiscience’s US partner of ES101, announced the dosing of first patient of ES101 in the US. With ES101 being tested in clinical trials both in China and the US, it is expected to benefit cancer patients worldwide.
Dr. Darren Ji, CEO of Elpiscience, said: “ES101 represents the arrival of the next generation of cancer immunotherapy beyond PD-1/PD-L1. As a first-in-class bispecific antibody, ES101 is expected to bring a transformative treatment to cancer patients on top of the existing therapies. We are delighted to witness this historical moment, which would have marked a small step for Elpiscience and its partner, and a giant leap for patients.”
About ES101
ES101 is a tetravalent bispecific antibody that contains four binding domains, two targeting PD-L1 and two targeting 4-1BB, with a molecular weight being only two-thirds of that of an conventional antibody. Based on the high expression of PD-L1 in the tumor microenvironment and an unique antibody engineering, ES101 can conditionally activate 4-1BB upon binding to PD-L1. This would allow for a more efficient tumor killing by immune cells.
About Elpiscience
Elpiscience is a clinical stage biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpscience’s first therapeutic product ES101 has entered into clinical trial, and the second innovative product ES102 is IND ready. Elpiscience has a pipeline of more than 10 innovative molecules in discovery, covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies. Founded and managed by seasoned executives in the biopharma industry, Elpiscience is backed by top investors such as Lilly Asia Ventures, Hillhouse Capital and CDH Investment.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Judy ShiJob descriptionJob description
Judy Shi is head of business development of Elpiscience. She has 20 years of experience in the pharmaceutical industry. Prior to joining Elpiscience, Judy served as BD Director at Wuxi AppTec’s Clinical Division. She was marketing manager at Roche China, Global Project Leader at Genentech, and worked for Xi’an Janssen and Schering-Plough China as a sales representative and product manager, respectively.
Judy holds a bachelor's degree in medicinal chemistry from Shenyang Pharmaceutical University in China.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
德屹资本Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
鼎晖投资Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Elpiscience Announces the Completion of Series A+ Financing of 35 Million USDJob descriptionJob description
Shanghai, 3rd December 2018. Elpiscience announced today that it has completed a 35 million USD series A+ round of financing led by Hillhouse Capital, with participation from CDH Investments and existing investor Lilly Asia Ventures (LAV). Together with 20 million USD raised in the series A round led by LAV, this financing brings the total funds to 55 million USD. Proceeds will be primarily used to advance its pipeline, including ES101 for which a Phase I clinical trial is planned to start in early 2019.
Elpiscience, has developed a pipeline of 12 R&D programs covering a wide range of targets with a particular focus on innate immunity and tumor microenvironment. Elpiscience’s sustainable pipeline forms a strong cornerstone for developing the next generation and more effective immunotherapies.
Dr. Darren Ji, co-founder and CEO of Elpiscience, said, “We extended our deepest thanks to Hillhouse, LAV and CDH for their support and recognition. They are certainly recognized as the best investors, have significant resources in healthcare industry with extensive experience, expertise, influence and strategic insight in China and global market. There is no doubt that endorsements from the top-tier investors definitely boost our value and step up our pace to be the most leading cancer immunotherapy company.”
About Elpiscience
Elpiscience is a top-tier investor backed biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment.
About Hillhouse
Hillhouse Capital is a global Asia-focused private equity firm founded by Lei Zhang in 2005. Hillhouse Capital is a global firm of investment professionals and operating executives who are focused on building and investing in high quality business franchises that achieve sustainable growth. Independent proprietary research and industry expertise, in conjunction with world-class operating and management capabilities, are key to Hillhouse Capital’s investment approach. Hillhouse Capital partners with exceptional entrepreneurs and management teams to create value, often with a focus on enacting technological transformation and innovation. Hillhouse Capital invests in the consumer, technology, media and telecommunications, healthcare, advanced manufacturing, financial and business services sectors in companies across all equity stages. For more information, please visit www.hillhousecap.com.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
InhibrxJob descriptionJob description
We have two partnered programs with Inhibrx, a bispecific antibody targeting 4-1BB and PD-L1 (ES101/INBRX-105) and an agonistic OX40 antibody (ES102/INBRX-106) in greater China.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Taoran WangJob descriptionJob description
Taoran Wang is general counsel of Elpiscience. Prior to joining Elpiscience, Taoran was a partner in Han Kun Law Offices, where specialized in several practice areas, including venture capital and private equity financings, mergers and acquisitions, capital market transactions, foreign direct investment, and general corporate matters.
Taoran received an LL.B. degree and an LL.M. degree from Southwest University of Political Science and Law, and also received a B.A. degree from Sichuan International Studies University. She also holds a PRC bar qualification.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Announces Filing of IND for ES101Job descriptionJob description
Shanghai, 2018.11.20. Elpiscience announced today that it has submitted an Investigational New Drug (IND) application to the Center of Drug Evaluation (CDE) in Beijing to support the initiation of a Phase I clinical trial of ES101 in China.
ES101, the most leading asset in Elpiscience R&D pipeline, is a first-in-class bispecific antibody targeting PD-L1 and 4-1BB, its PD-L1 dependent 4-1BB activation allows for robust anti-tumor immune activation and lower toxicity concerns in preclinical models.
Dr. Darren Ji, co-founder and CEO of Elpiscience, said “We are delighted to see that ES101, a promising immunotherapeutic bispecific antibody, meets a vital milestone, and we will work closely with the government authorities and clinical centers to move forward ES101 to clinical trials. Going forward, we plan to submit one to two IND applications each year, aspiring to continually bring novel medicines to serve the unmet medical needs.”
About ES101:
ES101 is a tetravalent bispecific antibody that contains four binding domains, two targeting PD-L1 and two targeting 4-1BB, with a molecular weight being only two-thirds of that of a conventional antibody. Based on high PD-L1 expression in the tumor microenvironment and ES101’s unique antibody engineering, ES101 can conditionally activate 4-1BB upon binding to PD-L1. This would allow for more efficient and targeted tumor killing by immune cells.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Peter Wang, MBAJob descriptionJob description
Peter is Vice President and Head of CMC of Elpiscience. He has nearly 20 years experience in process development and manufacturing in the US, Taiwan, and China with innovator companies KOSAN, Genentech, and Amgen, as well as CDMOs WuXi Biologics, CMAB, and JHL. He has strong hands-on experience in process development, manufacturing, and business development. Most recently, he supported the design, construction, commissioning, and operation of multiple GMP manufacturing facilities including Fed-Batch and Perfusion processes utilizing Ssingle-use and hybrid technologies.
Peter holds a bachelor’s degree in chemical engineering from the University of British Columbia and obtained his MBA from the University of Southern California.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
JunshiJob descriptionJob description
In May 2020 we entered a clinical collaboration with Junshi Biosicences to evaluate the safety, tolerability and potential early efficacy signal of ES102, a next generation OX40 agonistic antibody, in combination with toripalimab (TUOYI®), an anti-PD-1 mAb and immune checkpoint inhibitor for the treatment of patients with advanced solid tumors and hematological tumors.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience and Inhibrx Announce an Exclusive Collaboration and License Agreement of INBRX-106 (ES102) in Greater ChinaJob descriptionJob description
Shanghai and San Diego, May 1st 2018. Elpiscience Biopharmaceutical Ltd (Elpiscience) has entered into a licensing agreement with Inhibrx, Inc., pursuant to which Elpiscience was granted an exclusive right to develop and commercialize INBRX-106 (ES102) in mainland China, Hongkong, Macau and Taiwan.
ES102 is a hexavalent OX40 agonistic antibody with a unique “first-in-class” design. The special design of ES102 allows for efficient OX40 agonism even in the absence of exogenous cross-linking. ES102 induces much strong immune activation compared to tetrameric or conventional bivalent OX40 antibodies.
About ES102
ES102 is a hexavalent OX40 agonistic antibody with best-in-class potential. ES102 has shown impressive anti-tumor efficacy as monotherapy in pre-clinical models compared to conventional bivalent antibodies and has the potential to translate clinically. OX40, also known as CD134, is a member of the tumor necrosis factor (TNF) receptor family and is an important co-stimulator of T cell responses. Typically for TNF receptor family members, 3 molecules of OX40 bind to the trimeric OX40 ligand protein, activating downstream NF-κB, PI3K, and AKT pathways, which ultimately leads to increased cytokine production and is associated with enhanced T-cell expansion, differentiation, and the generation of long-lived memory T-cells.
About Elpiscience
Elpiscience is a top-tier investor backed biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment.
About Inhibrx
Inhibrx is a clinical-stage biotechnology company focused on developing a broad pipeline of novel biologic therapeutic candidates. Inhibrx utilizes diverse methods of protein engineering to address the specific requirements of complex target and disease biology, including its proprietary sdAb platform. The Inhibrx pipeline is focused on oncology, orphan diseases and infectious diseases. Inhibrx has collaborations with Celgene and bluebird bio and has received awards from several granting agencies, including NIH, NIAID and CARB-X.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Grelia Zhang, EMBAJob descriptionJob description
Grelia Zhang is the Head of Human Resources at Elpiscience, with more than 17 years of comprehensive experience across all domains of HR management.
Prior to joining Elpiscience, Grelia served as Associate Director of Human Resources at Gilead Sciences, where she supported the company’s rapid business expansion in China and played a key strategic role in organizational evolution and talent deployment.
Before Gilead, she held several key HR positions at Roche China, spanning both functional HR teams and HR business partner roles. She provided HR leadership for diverse business areas, including commercial operations and manufacturing. In addition, she served as a Global Leadership Development Project Manager at Roche.
Grelia holds a Bachelor’s degree in Electrical Engineering from Shanghai Institute of Technology and a Master’s degree in Industrial Economics from Shanghai Maritime University. She also earned a Global EMBA from China Europe International Business School (CEIBS).
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Technical OperationsJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Novel BiME Molecule for Depletion of B Cells and Other immune cellsJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Elpiscience and Inhibrx Announce an Exclusive Collaboration and License Agreement of INBRX-105 (ES101) in Greater ChinaJob descriptionJob description
Shanghai and San Diego, March 01st 2018. Elpiscience Biopharmaceutical, Ltd. (Elpiscience), a biotech company focusing on the discovery and development of next-generation cancer immunotherapies, announced today that it entered into an exclusive licensing agreement with Inhibrx, Inc., to develop and commercialize its tetravalent bi-specific antibody that targets PD-L1 and 4-1BB, also referred to as INBRX-105 or ES101, in mainland China, Hongkong, Macau and Taiwan.
ES101, as a first-in-class bispecific antibody, has the potential to become the next-generation immunotherapeutic drug. It was designed based on the concept of “de-brake and add gas” for immune cell activation. De-braking is accomplished by blocking PD-1/PD-L1 checkpoint inhibitor interaction, whilst adding gas is achieved by 4-1BB-mediated immune cell engagement and activation.
About ES101:
ES101 is a tetravalent bispecific antibody that contains four binding domains, two targeting PD-L1 and two targeting 4-1BB, with a molecular weight being only two-thirds of that of a conventional antibody. Based on high PD-L1 expression in the tumor microenvironment and ES101’s unique antibody engineering, ES101 can conditionally activate 4-1BB upon binding to PD-L1. This would allow for more efficient and targeted tumor killing by immune cells.
About Elpiscience:
Elpiscience is a top-tier investor backed biopharmaceutical company focusing on cancer immunotherapy. The company is committed to leading the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience’s innovations cover a wide range of immunology targets with a focus on innate immunity and tumor microenvironment.
About Inhibrx:
Inhibrx is a clinical-stage biotechnology company focused on developing a broad pipeline of novel biologic therapeutic candidates. Inhibrx utilizes diverse methods of protein engineering to address the specific requirements of complex target and disease biology, including its proprietary sdAb platform. The Inhibrx pipeline is focused on oncology, orphan diseases and infectious diseases. Inhibrx has collaborations with Celgene and bluebird bio and has received awards from several granting agencies, including NIH, NIAID and CARB-X.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Tesla, Inc. 2021 Annual Shareholder MeetingJob descriptionJob description
Tesla, Inc. 2021 Annual Shareholder Meeting,Text description to be provided...
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Hongtao Lu, Ph.D.Job descriptionJob description
Hongtao is Co-founder of Elpiscience. Trained as an immunologist, Hongtao is a 20-year industry veteran, with R&D experience developing both small molecule and biologic-based therapies across several therapeutic areas, including autoimmune diseases, oncology, and neuroscience. Before co-founding Elpiscience, Hongtao co-founded Zai Lab, a NASDAQ-listed company, as its Vice President and helped to build a robust pipeline focusing on oncology and autoimmune diseases. Prior to Zai Lab, he was with GlaxoSmithKline (GSK) as the founding head and senior director of Neuroimmunology Discovery Performance Unit (NI-DPU), where he led a team of 70 biologists and chemists focusing on drug discovery in multiple sclerosis and other neurodegenerative diseases including Parkinson's disease. While at GSK, he also successfully built this R&D team and the pipeline in only 5 years, producin three clinical-stage assets and four pre-clinical programs.
Dr. Lu spent his early career with Berlex Biosciences and Bayer Schering Pharma, where he conducted target identification and validation in autoimmune inflammatory diseases and oncology. Dr. Lu holds a Ph.D. in Regulatory Biology and conducted his graduate research at the Cleveland Clinic Foundation, focusing on MHC class II gene regulation. After receiving his doctorate, Dr Lu completed his post-doctoral fellowship at Yale University School of Medicine under the guidance of Dr. Richard Flavell, where he worked on T cell differentiation and signal transduction pathway.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Shirley Tu, AICPAJob descriptionJob description
Shirley Tu is the Head of Operations and Finance at Elpiscience, responsible for the Company’s overall operations and financial management.
She began her career in 2007 at the Jiangxi Branch of the Bank of China and subsequently moved into accounting roles at Mok Accountancy Corporation and Shanghai ETL Accounting Firm. From 2014 to 2015, Shirley served as a Tax Consultant at PricewaterhouseCoopers, followed by her role as Finance Manager at Nemetschek Software Engineering (Shanghai) Co., Ltd. from 2015 to 2017.
Shirley holds a Bachelor’s degree in Accounting from Zhongnan University of Economics and Law and a Master’s degree in Accounting with distinction from Golden Gate University. She has been a Certified Public Accountant in the State of California since 2014.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Cell Line DevelopmentJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Elpiscience Announces Inauguration in ZhangjiangJob descriptionJob description
Shanghai, October 8, 2017. Elpiscience, a start-up biopharma focusing on cancer immunotherapies, announces that it officially starts the operation in Zhangjiang High-Tech Park. Elpiscience was founded in June and raised 20 million USD in series A financing, which could be used to support its operation.
Elpiscience gathers a group of business elites and research talents, intending to lead the discovery and development of the next-generation cancer immunotherapies based on its deep understanding of immunology and cancer biology, as well as its world class antibody development capabilities.
Zhangjiang High-tech Park is the most advanced innovation center all over the China, which has formed an excellent framework of biomedical innovation chain and a high-quality environment for developing biopharmaceutical industry. Based on deep pool of professional leading talents and the innovative and entrepreneurial atmosphere, Elpiscience is anticipated to boost rapidly to become one of the most promising cancer immunotherapy company across the world.
About Elpiscience
Elpiscience was established in June 2017 with the commitment to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience was founded by a seasoned team that combined management talents and R&D abilities together, aiming to build a robust and sustainable IO pipeline to tackle the unmet clinical needs. Elpiscience is backed by top investor Lilly Asia Ventures.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
OwnershipJob descriptionJob description
People at Elpiscience are owners of the company. They are self-motivated and fully empowered to make the right decisions. They take accountabilities for outcomes attributed to their work and the work of their team.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Story Title SpaceJob descriptionJob description
Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided,Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided,Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided。
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Shanghai Harnessing Immunology
to Fight CancerJob descriptionJob description
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Tesla, Inc. 2021 Annual Shareholder MeetingJob descriptionJob description
Tesla, Inc. 2021 Annual Shareholder Meeting,Text description to be provided...
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Darren Ji, MD, Ph.D., MBAJob descriptionJob description
Darren is Co-founder, Chairman, and CEO of Elpiscience. Prior to co-founding Elpiscience, Darren served as Venture Partner of Lilly Asia Ventures (LAV). Before joining LAV, Darren served as the Global Head and Vice President for Asia and Emerging Markets of Roche Partnering, Roche’s deal-making body that manages the company’s business development. While at Roche, Darren was responsible for driving the strategy and execution of partnering activities in the territory of Asia and Emerging Markets encompassing over 100 countries. During his tenure, Darren also championed and oversaw the closing of many key transactions between Roche and partners worldwide. He managed a global team and established a strong business network in key countries like China, Japan, Korea, Australia/New Zealand, Russia, and Brazil.
An accomplished entrepreneur himself, Darren spent a long career at the Procter & Gamble Company with increasing responsibilities in drug R&D and business development. He co-founded and managed as CEO of PharmaLegacy Laboratories in Shanghai in 2008, which became a premium CRO providing high-quality drug discovery services to a global clientele. Darren has been a highly respected leader in global life sciences and a sought-after speaker in various business forums. He was also an avid community builder exemplified by one of the longest tenures as a board member of the BayHelix Group.
Darren received his medical training at China Medical University, a Ph.D. from the University of Sheffield in the UK, and an MBA from the University of Chicago.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
BiME® - Bispecific Macrophage EngagerJob descriptionJob description
BiME®: Unlocking the Power of Macrophages in Cancer Immunotherapy
What is BiME®?BiME® (Bispecific Macrophage Engager) is a groundbreaking bispecific antibody-based platform designed to activate macrophages and enhance their tumor-killing power.Tumor cells evade immune destruction by exploiting a “don’t-eat-me”signal which interacts with SIRPα on macrophages to block phagocytosis. BiME® disrupts this mechanism while simultaneously directing macrophages to attack tumors.How BiME® Works? ✔ Blocks the “don’t-eat-me” signal – The SIRPα-targeting antibody prevents CD47 from sending inhibitory signals to myeloid.✔ Delivers an “eat-me” signal – The tumor-associated antigen (TAA)-targeting antibody binds to cancer cells, activating myeloid cells through Fc receptor engagement.✔ Boosts tumor cell phagocytosis – Triggers strong myeloid cell phagocytosis leading to direct tumor cell elimination.✔ Reprograms the TME – Converts suppressive TAMs into M1-like macrophages for a sustained anti-tumor response.✔ Up to phagocytosis, myeloid cells present tumor antigens to T cells activating T cells which leads to eventual tumor killing.With its unique dual-targeting approach, BiME® turns tumors from immune-resistant (“cold”) into immune-responsive (“hot”), unlocking new treatment possibilities.Why is BiME® a Game-Changer?Broad Applications Across Solid and Blood CancersBiME® technology is designed to address a wide range of malignancies, offering therapeutic potential across both solid tumors and hematologic cancers.Enhanced Safety ProfileUnlike many T-cell therapies, BiME® significantly reduces the risk of cytokine storms—one of the major safety concerns associated with T-cell engager (TCE)-based treatments.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
✔ Blocks the “don’t-eat-me” signal – The SIRPα-targeting antibody prevents CD47 from sending inhibitory signals to myeloid.✔ Delivers an “eat-me” signal – The tumor-associated antigen (TAA)-targeting antibody binds to cancer cells, activating myeloid cells through Fc receptor engagement.✔ Boosts tumor cell phagocytosis – Triggers strong myeloid cell phagocytosis leading to direct tumor cell elimination.✔ Reprograms the TME – Converts suppressive TAMs into M1-like macrophages for a sustained anti-tumor response.✔ Up to phagocytosis, myeloid cells present tumor antigens to T cells activating T cells which leads to eventual tumor killing.With its unique dual-targeting approach, BiME® turns tumors from immune-resistant (“cold”) into immune-responsive (“hot”), unlocking new treatment possibilities.Why is BiME® a Game-Changer?Broad Applications Across Solid and Blood CancersBiME® technology is designed to address a wide range of malignancies, offering therapeutic potential across both solid tumors and hematologic cancers.Enhanced Safety ProfileUnlike many T-cell therapies, BiME® significantly reduces the risk of cytokine storms—one of the major safety concerns associated with T-cell engager (TCE)-based treatments.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Darren Ji, MD, Ph.D., MBAJob descriptionJob description
Darren is Co-Founder, Chairman, and CEO of Elpiscience. Prior to co-founding Elpiscience, Darren served as Venture Partner of Lilly Asia Ventures (LAV). Before joining LAV, Darren served as the Global Head and Vice President for Asia and Emerging Markets of Roche Partnering, Roche’s deal-making body that manages the company’s business development. While at Roche, Darren was responsible for driving the strategy and execution of partnering activities in the territory of Asia and Emerging Markets encompassing over 100 countries. During his tenure, Darren also championed and oversaw the closing of many key transactions between Roche and partners worldwide. He managed a global team and established a strong business network in key countries like China, Japan, Korea, Australia/New Zealand, Russia, and Brazil.
An accomplished entrepreneur himself, Darren spent a long career at the Procter & Gamble Company with increasing responsibilities in drug R&D and business development. He co-founded and managed as CEO of PharmaLegacy Laboratories in Shanghai in 2008, which became a premium CRO providing high-quality drug discovery services to a global clientele. Darren has been a highly respected leader in global life sciences and a sought-after speaker in various business forums. He was also an avid community builder exemplified by one of the longest tenures as a board member of the BayHelix Group.
Darren received his medical training at China Medical University, a Ph.D. from the University of Sheffield in the UK, and an MBA from the University of Chicago.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
内容在这Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Cell Culture Process DevelopmentJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Alex Lin, MBAJob descriptionJob description
Alex is Chief Financial Officer of Elpiscience. Alex has over 14 years of experience across private equity, investment banking, and Fortune 500 companies.
Prior to joining Elpiscience, Alex was Managing Director at CBC Group, focusing on investments, acquisitions, and post-investment management in healthcare industry. Prior to CBC, he spent most of his investment banking career in the Global Healthcare Group at BofA Securities (previously known as BofA Merrill Lynch) in its New York office, later transferring to its Hong Kong office to cover Greater China healthcare clients. Earlier, he worked at Procter & Gamble and Johnson & Johnson MedTech.
Alex holds an MBA from The Tuck School of Business at Dartmouth College, and a bachelor's degree in engineering from Huazhong University of Science and Technology.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Elpiscience Completes Series A Financing of 20 Million USDJob descriptionJob description
Shanghai, August 30, 2017. Elpiscience announces it has raised 20 million USD in series A financing, invested by Lilly Asia Ventures (LAV). Elpiscience intends to use the proceeds from the financing to initiate operation, expand IO pipeline and establish its own R&D platforms.
Elpiscience, established in June 2017 by Darren Ji, Hongtao Lu, and David Shen, is committed to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Leveraging the deep understanding of immunology and with its highly efficient execution capabilities, Elpiscience will focus on the most frontier targets in innate and adaptive immunities, and building a world-class pipelines in cancer treatment.
Dr. Darren Ji, co-founder and CEO of Elpiscience, said “We are delighted to be supported by one of the most leading biomedical venture capital firms at the beginning of our journey. I am on behalf of Elpiscience to express our deep attitude for LAV’s recognition and trust. We are thrilled to strive with like-minded partners to be the best possible immunotherapy biopharma company that could be.”
About Elpiscience
Elpiscience was established in June 2017 with the commitment to leading the innovation and development of the next generation of cancer immunotherapies for the benefit of cancer patients worldwide. Elpiscience was founded by a seasoned team that combined management talents and R&D abilities together, aiming to build a robust and sustainable IO pipeline to tackle the unmet clinical needs.
About Lilly Asia Venture (LAV)
Lilly Asia Ventures (LAV) is a leading biomedical venture capital firm, with offices in Shanghai, Hong Kong, and Palo Alto. Originated in 2008 as a corporate venture subsidiary of Eli Lilly, we spun off and became an independent investment management company. As one of the earliest biomedical venture investors in China, we have been consistently investing in the region for over a decade, and have recently increased our footprint in the U.S. Our team of over 30 distinguished scientific, medical, investment, and operational professionals embrace a culture of integrity, entrepreneurship, and team work. Currently, we manage over $1.2 billion of committed capital. LAV has significant resources in China, with extensive local expertise in preclinical and clinical development, regulatory knowhow, and market insights.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
CourageJob descriptionJob description
People at Elpiscience have the courage to take risks and always explore the best way to have the work done. They communicate transparently and are willing to speak up, and are never afraid of challenging the status quo.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Story Title Space2Job descriptionJob description
Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided,Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided,Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided。
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Suzhou Harnessing Immunology
to Fight CancerJob descriptionJob description Room 206, A3, Creative Industrial Park, 328 Xinghu Street, SIP, Jiangsu, China 215123
Room 206, A3, Creative Industrial Park, 328 Xinghu Street, SIP, Jiangsu, China 215123 +86 512 83981611
+86 512 83981611 info@elpiscience.com
info@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Tesla, Inc. 2021 Annual Shareholder MeetingJob descriptionJob description
Tesla, Inc. 2021 Annual Shareholder Meeting,Text description to be provided...
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Announcement of semi annual performance express in 2020Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Acebody™ - H - L Interchain Disulfide Bond Engineering BsAb PlatformJob descriptionJob description
Acebody™, Generate Better IgG-like Bispecific Antibody in Less Time
Asymmetric architecture is one of the widely used formats of bispecific therapeutic antibodies. This type of format is comprised of four different chains (H1, H2, L1 and L2), resulting in 10 different combinations, among which only one molecule can be selected for development. If not engineered smartly during the antibody generation process, challenges will arise at the CMC stage for the molecule in its purification, production volume, and ultimately, the total time investment will increase considerably.
What is Acebody™?
Acebody™ is an H-L interchain disulfide bond engineering platform that can solve the challenging issue for IgG-like BsAb by adopting 2 pairs of alternative interchain disulfide bond to increase cognate H-L pairing rate and stability of the target antibody. It combines the Knock-into-Hole design in the heavy chain, and transfers two antibodies to an IgG-like bispecific antibody through a simple purification process without impairing the desired affinity and property. More importantly, the purity level is preserved by more than 97%, compared to the parental antibodies, without any light chain mismatching after going through the purification with protein A.
Gain Superiority through Acebody™- Compared to other bispecific antibody formats, IgG-like structure retains the native architecture of natural antibodies as closely as possible to preserve the associated functional characteristics and favorable quality attributes.
- Simplified production workflow: Protein A + IEX
- Good developability: Low aggregation tendency, high thermo stability, and easy to scale up
- Alternative cysteine technology (Ace) to facilitate cognate heavy and light (HL) chain pairing.
- Knob-into-Hole approach to promote heavy chain hetero-dimerization.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES102Job descriptionJob description
ES102: Potential Best-in-Class OX40 Agonist Antibody
OX40 (CD134) is a member of the tumor necrosis factor (TNF) receptor family and is an important co-stimulator of T cell responses. Three OX40 molecules bind to the trivalent OX40 ligand (OX40L), activating downstream NF-κB, PI3K, and AKT pathways, which leads to an increased cytokine production. The activation of OX40 enhances T cell expansion, differentiation, and the generation of memory T cells.ES102 is a hexavalent OX40 agonist that consists of six OX40 single-domain antibody structures, which facilitates the clustering of OX40. ES102 induces a stronger downstream signaling than bivalent OX40 antibodies and trivalent endogenous OX40L.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Regulatory AffairsJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Purification DevelopmentJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Elpiscience Announces the Establishment of Board of DirectorsJob descriptionJob description
Shanghai, June 25, 2017. Elpiscience has established its board of directors and held the first meeting in Shanghai. Board members include Darren Ji (co-founder, CEO), Hongtao Lu (co-founder, CSO), David Shen (Chairman of SAB), Hongbo Lu (Lilly Asia Venture) and Yi Shi (Lilly Asia Venture).

Cancer is still commonly recognized as a global burden that many types of cancer unfortunately lack an effective solution. Through the efforts of several generations, medical scientists have brought forth tremendous changes to the landscape of cancer management, from surgery to subsequent radio and chemo therapy, then the R&D of targeted therapy in late 20s. The recently epochal turn took place in 2010 with the application ofimmune checkpoints inhibitors for treatment of multiple tumors. Immuno-oncology (IO) treatment, hailed as one of the most unique and promising cancer treatments, are rapidly developed resulting from the positive clinical benefit shown by checkpoint inhibitors (PD-1/L1…), considered the first wave of IO therapies. However, there are around 70%-80% non-responders of total tumor patients remain undeserved.
In order to develop more effective therapies for cancer, Elpiscience was founded to focus on critical biology that would provide viable intervention points to bring clinical benefits. The establishment of Board of Directors marked that Elpiscience was ready to sail with the commitment to leading the innovation and development of the next generation of cancer immunotherapies.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
RigorJob descriptionJob description
People at Elpiscience are passionate about achieving the highest level of quality in everything we do. We do not take any short-cuts. This is the key for us to discover and develop successful new medicines with high quality.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Story Title SpaceJob descriptionJob description
Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided,Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided,Story content description space to be provided Story Story content description space to be provided Story content description space to be provided content description space to be provided。
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Investor Contact InformationJob descriptionJob description
 phone: (+86)2150651310
phone: (+86)2150651310 mailbox: info@elpiscience.comIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
mailbox: info@elpiscience.comIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Financial report of 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Tesla, Inc. 2021 Annual Shareholder MeetingJob descriptionJob description
Tesla, Inc. 2021 Annual Shareholder Meeting,Text description to be provided...
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Announcement of semi annual performance express in 2021Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Announcement of semi annual performance express in 2020Job descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Our VisionJob descriptionJob description
Advancing immunotherapy to transform lives with the power of science.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Our MissionJob descriptionJob descriptionTo develop innovative and transformative immunotherapies that harness the power of the immune system to fight cancer and autoimmune diseases.If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Core ValuesJob descriptionJob description
Find a job you love, and you will never have to work a single day.
— Confusius
The Elpiscience team spent 2 and 1/2 days in Ningbo to discuss our company strategy and enjoy many fun activities together. Watch the video to see our matrix team in full action. Come and join us if you want to become a member of a team dedicated to Discovery and Development of innovative biotherapeutic medicines for cancer patients in need.
There are three things that we value at Elpiscience: Passion, Leadership, and Having Fun.
Passion drives our curiosity to constantly seek answers. It takes us from dreams to realizable goals and to the sky as the limit. Passion makes us exceptional team members that work together to create wonders that wouldn’t be achievable otherwise.
Leadership is in our blood. We lead scientific discoveries and will not settle with a “me too”. We lead as people always seeking to be part of a solution, not a part of a problem. We lead to initiate, to own, to collaborate, and to deliver as true leaders do.
We believe in having fun at the workplace. Having fun transforms an idea into an achievement. It turns a job into a career, and an individual into passionate leader. We create fun; we have fun!
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to operations@elpiscience.com
 If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Immuno-Oncology ApproachJob descriptionJob descriptionElpiscience is dedicated to transforming cancer treatment by developing innovative immunotherapies that turn “cold” tumors “hot”. By unlocking differentiated biology, we aim to enhance crosstalk between innate and adaptive immunity, harnessing the full potential of the immune system to fight cancer .Cancer ImmunotherapyHarnessing the immune system to fight cancer has been a scientific pursuit for over a century. In recent years, immune checkpoint inhibitors have revolutionized cancer treatment, offering patients powerful new options.However, two key challenges remain: identifying novel biological pathways with clinical relevance and developing preclinical models that reliably predict therapeutic success.At Elpiscience, we tackle these challenges by uncovering unique biological insights that can effectively activate the immune system. Our experienced research and development teams, supported by world-renowned scientists, are committed to advancing next-generation immunotherapies.Our Systemic Dual ApproachTo overcome the immune system’s failure to fight cancer, Elpiscience is committed to revolutionizing cancer treatment by adopting a holistic and systematic approach to immunotherapy. Since multiple mechanisms contribute to immune evasion, we focus on two key strategies to restore immune function and drive robust anti-tumor responses.
Removing Immune Suppressive Factors in the Tumor Microenvironment (TME)
Tumors create an immunosuppressive microenvironment that actively inhibits immune responses. Various immune and non-immune cells within the TME secrete factors that trigger inhibitory signals, forming a significant barrier to effective immunotherapy. Elpiscience develops targeted therapies to neutralize these suppressive mechanisms, enhancing the immune system’s ability to fight cancer.
Reinvigorating Exhausted T Cells
In cancer patients, prolonged antigen exposure leads to T cell exhaustion, reducing their ability to attack tumors. Restoring T cell function is essential for a sustained and potent immune response. Elpiscience designs therapies that modulate costimulatory and inhibitory signaling pathways, revitalizing T cells to generate stronger and more durable anti-tumor effects.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ElpiSource™ - Human Antibody Fab LibraryJob descriptionJob description
ElpiSource™, Rapid Generation of Desirable Fully Human Antibody
Antibody discovery generally involves animal immunization, antibody screening by hybridoma or displaying, and humanization. It normally takes 5-7 months to complete and is not very efficient. Speed and efficiency in drug discovery is very important in a fast-changing market. Using a fully human antibody library for antibody generation can accelerate the process and generate therapeutic leads without the need of humanization.
What is ElpiSource™?
ElpiSource is a human naïve Fab antibody library displayed on yeast cell surface with diversity exceeding 5xE10. The library reduces the time for "target to sequence" delivery to 2-3 months by skipping the process for animal immunization and humanization. It avoids the potential risk of affinity change derived from humanization and format conversion. More unique sequences can be generated.
The library contains a semi-synthetic sub-library that covers main human productive heavy chain germline frameworks and a completely naïve sub-library that captures natural diversity.
It uses flow cytometry to manipulate the sorting strategies. It is compatible for both cell-based and protein-based screening, and can be customized for multiple types of antibodies.
By employing flow cytometry and yeast surface display, we have developed a series of methods to predict antibody developability, and can monitor and select the clones in real-time with the desired properties, such as expression level, stability, binding affinity, and specificity, et al.
 If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
ES104Job descriptionJob description
ES104: Potential First-in-Class Anti-VEGFxDLL4 Bispecific Antibody
ES104 is a bispecific antibody targeting both vascular endothelial growth factor (VEGF) and Delta-like ligand 4/Notch (DLL4), two critical pathways driving angiogenesis and tumor vascularization. VEGF blockade reduces tumor vessel formation, which is synergistically enhanced by DLL4 inhibition, leading to decreased vessel perfusion. Dual blockade of VEGF and DLL4 is designed to overcome multi-VEGF resistance.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Translational MedicineJob descriptionJob description
Elpiscience’s translational platform integrates cutting-edge immunobiology, PK/PD analysis, and multi-omics data to optimize drug development for tumor immunotherapy and autoimmune diseases. Our in vitro pharmacodynamics, DMPK & toxicology, and biomarker platforms ensure mechanism-driven research, precise dose selection, and biomarker-driven therapies. Through a science-first, data-driven approach, we accelerate the development of safe and effective breakthrough treatments.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Clinical ScienceJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Technical OperationJob descriptionIf you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com
-
Our Leadership CommitmentJob descriptionJob description
- I strive to be a role model in demonstrating our core values
- I recoganize and unleash the potential in our people
- I empower and trust our people to do the right thing
- I inspire our people to deliver to the fullest
- I listen, communicate effectively, and explain why
- I have the courage to make decisions
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
Our Talent StrategyJob descriptionJob description
We aim to attract top global talents and commit to investing in developing talent. We empower and trust our colleagues to own their career. We support colleagues to develop vertically and horizontally. You can be developed from a junior scientist to a senior principal or from a first line manager to a senior leader. You can also extend your skill-sets across the organization if you are willing to learn and do new things. We provide opportunities to help our talents to step out of their comfortable zones, to unleash their potentials and become better selves.
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com -
USA DiscoveryJob descriptionJob description
 12410 Milestone Center Drive, Suite 600, Germantown, MD, USA 20876
12410 Milestone Center Drive, Suite 600, Germantown, MD, USA 20876
If you are a like-minded person who seeks challenges and excitement, we invite you to join us. Please submit your CV and enquiries to joinus@elpiscience.com